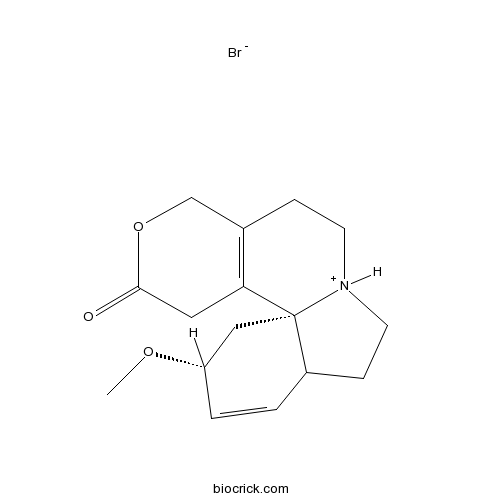
Dihydro-β-erythroidine hydrobromideantagonist of nAChRs CAS# 29734-68-7 |
- Clinafloxacin CI96 AM1091
Catalog No.:BCC3754
CAS No.:105956-97-6
- Voreloxin Hydrochloride
Catalog No.:BCC2045
CAS No.:175519-16-1
- Delafloxacin meglumine
Catalog No.:BCC1523
CAS No.:352458-37-8
- Amonafide
Catalog No.:BCC1249
CAS No.:69408-81-7
- Sarafloxacin HCl
Catalog No.:BCC4713
CAS No.:91296-87-6
- Enrofloxacin
Catalog No.:BCC4657
CAS No.:93106-60-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29734-68-7 | SDF | Download SDF |
| PubChem ID | 34682 | Appearance | Powder |
| Formula | C16H22BrNO3 | M.Wt | 356.26 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DHβE | ||
| Solubility | Soluble to 100 mM in water and to 25 mM in DMSO | ||
| SMILES | COC1CC23C(CC[NH+]2CCC4=C3CC(=O)OC4)C=C1.[Br-] | ||
| Standard InChIKey | ITZHVVLSHDXSIJ-WDXUOIDCSA-N | ||
| Standard InChI | InChI=1S/C16H21NO3.BrH/c1-19-13-3-2-12-5-7-17-6-4-11-10-20-15(18)8-14(11)16(12,17)9-13;/h2-3,12-13H,4-10H2,1H3;1H/t12?,13-,16-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Competitive nicotinic acetylcholine receptor antagonist with moderate selectivity for the neuronal α4 receptor subunit (IC50 values are 0.19 and 0.37 μM for α4β4 and α4β2 receptors respectively). Antagonizes behavioral effects of nicotine in vivo. |

Dihydro-β-erythroidine hydrobromide Dilution Calculator

Dihydro-β-erythroidine hydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8069 mL | 14.0347 mL | 28.0694 mL | 56.1388 mL | 70.1735 mL |
| 5 mM | 0.5614 mL | 2.8069 mL | 5.6139 mL | 11.2278 mL | 14.0347 mL |
| 10 mM | 0.2807 mL | 1.4035 mL | 2.8069 mL | 5.6139 mL | 7.0173 mL |
| 50 mM | 0.0561 mL | 0.2807 mL | 0.5614 mL | 1.1228 mL | 1.4035 mL |
| 100 mM | 0.0281 mL | 0.1403 mL | 0.2807 mL | 0.5614 mL | 0.7017 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 1.3 μM for α2β2, 2.3 μM for α2β4, 0.41 μM for α3β2, 23.1 μM for α3β4, 0.37 μM for α4β2, and 0.19 μM for α4β4 [1]
Dihydro-β-erythroidine hydrobromide (DHβE), the hydrogenated derivative of erythroidine, is a competitive antagonist of neuronal nicotinic acetyicholine receptors (or nAChRs). Nicotinic acetyicholine receptors are neuron receptor proteins which respond to the neurotransmitter acetylcholine.
In vitro: DHβE has been shown to be a purely competitive antagonist of the neuronal nicotinic receptor [1].
In vivo: DHβE is able to block some of the central actions of nicotine after systemic and intrathecal administration. The mechanism of blockade is different from that of mecamylamine, a classical ganglionic antagonist, and may involve a direct action of DHβE on nicotine receptor [2].
Clinical trial: DHβE can be given orally and may cross the blood-brain barriers. At 200 mg/kg, the effects were bradycardia and visual difficulty most often described as blurring of vision or double vision; at 6 mg/kg, produced aforementioned effects plus hypotension and reduction in grip strength with accompanying feelings of sedation [3].
References:
[1] Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. J Neurochem. 1996 Nov;67(5):1953-9.
[2] Damaj MI, Welch SP, Martin BR. In vivo pharmacological effects of dihydro-beta-erythroidine, a nicotinic antagonist, in mice. Psychopharmacology (Berl). 1995 Jan;117(1):67-73.
[3] MURPHREE HB. Effects in human volunteers of subparalytic doses of dihydro-beta-erythroidine. Clin Pharmacol Ther. 1963 May-Jun;4:304-10.
- Clopidol
Catalog No.:BCC8918
CAS No.:2971-90-6
- Methyl chlorogenate
Catalog No.:BCC9042
CAS No.:29708-87-0
- DOB hydrochloride
Catalog No.:BCC5947
CAS No.:29705-96-2
- dihydrokaempferol
Catalog No.:BCC8191
CAS No.:5150-32-3
- Oxyresveratrol
Catalog No.:BCN5201
CAS No.:29700-22-9
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- 2-Aminothiazol-4-acetic acid
Catalog No.:BCC8556
CAS No.:29676-71-9
- 13-Oxo-9E,11E-octadecadienoic acid
Catalog No.:BCN8173
CAS No.:29623-29-8
- Friedelin 3,4-lactone
Catalog No.:BCN6449
CAS No.:29621-75-8
- Gynuramide II
Catalog No.:BCN5200
CAS No.:295803-03-1
- 2-Amino-2',5-dichlorobenzophenone
Catalog No.:BCC8520
CAS No.:2958-36-3
- Sakuranetin
Catalog No.:BCN5199
CAS No.:2957-21-3
- Apigenin-7-glucuronide
Catalog No.:BCN5326
CAS No.:29741-09-1
- Luteolin-7-O-glucuronide
Catalog No.:BCN5338
CAS No.:29741-10-4
- Loganetin
Catalog No.:BCN5202
CAS No.:29748-10-5
- Altretamine hydrochloride
Catalog No.:BCC4114
CAS No.:2975-00-0
- Altenuene
Catalog No.:BCN7392
CAS No.:29752-43-0
- Teniposide
Catalog No.:BCC3864
CAS No.:29767-20-2
- Nudifloside B
Catalog No.:BCN7474
CAS No.:297740-98-8
- Nudifloside C
Catalog No.:BCN7491
CAS No.:297740-99-9
- Silydianin
Catalog No.:BCN2388
CAS No.:29782-68-1
- Carbamazepine
Catalog No.:BCC4378
CAS No.:298-46-4
- Threo-methylphenidate hydrochloride
Catalog No.:BCC5818
CAS No.:298-59-9
- Xanthotoxin
Catalog No.:BCN5205
CAS No.:298-81-7
Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits.[Pubmed:8863500]
J Neurochem. 1996 Nov;67(5):1953-9.
Neuronal nicotinic acetylcholine receptors are differentially sensitive to blockade by the competitive antagonist dihydro-beta-erythroidine. Both alpha and beta subunits participate in determining sensitivity to this antagonist. The alpha subunit contribution to dihydro-beta-erythroidine sensitivity is illustrated by comparing the alpha 4 beta 4 receptor and the alpha 3 beta 4 receptor, which differ in sensitivity to dihydro-beta-erythroidine by approximately 120-fold. IC50 values for blocking alpha 4 beta 4 and alpha 3 beta 4, responding to EC20 concentrations of acetylcholine, were 0.19 +/- 0.06 and 23.1 +/- 10.2 microM, respectively. To map the sequence segments responsible for this difference, we constructed a series of chimeric alpha subunits containing portions of the alpha 4 and alpha 3 subunits. These chimeras were coexpressed with beta 4, allowing pharmacological characterization. We found determinants of dihydro-beta-erythroidine sensitivity to be distributed throughout the N-terminal extracellular domain of the alpha subunit. These determinants were localized to sequence segments 1-94, 94-152, and 195-215. Loss of determinants within segment 1-94 had the largest effect, decreasing dihydro-beta-erythroidine sensitivity by 4.3-fold.
In vivo pharmacological effects of dihydro-beta-erythroidine, a nicotinic antagonist, in mice.[Pubmed:7724704]
Psychopharmacology (Berl). 1995 Jan;117(1):67-73.
The comparative in vivo pharmacology of mecamylamine and dihydro-beta-erythroidine (DH beta E) in mice was studied. Modulation of the behavioral effects (antinociception, hypomotility, motor impairment and hypothermia) of nicotine in mice by DH beta E and mecamylamine were carried out. After SC administration, DH beta E and mecamylamine were nearly equipotent in blocking nicotine's effects except for antinociception, in which mecamylamine was clearly more potent. Intrathecal injection of DH beta E was also effective in blocking the antinociceptive effect of nicotine. In vivo interaction of DH beta E with calcium and calcium channels, involved in the central actions of nicotine, showed that intrathecal administration of DH beta E failed to reduce the antinociception induced by diverse drugs which increase intracellular calcium such as thapsigargin, (+/-)-BAYK 8644 and calcium, indicating that this antagonist does not affect calcium-dependent mechanisms involved in antinociception. On the other hand, mecamylamine blocked the antinociceptive effect of the calcium modulatory drugs, suggesting that it may be acting on calcium-dependent mechanisms involved in the intracellular signaling process. We conclude that DH beta E, a nicotinic neuromuscular antagonist, is able to block some of the central actions of nicotine after systemic and intrathecal administration. The mechanism of blockade is different from that of mecamylamine, a classical ganglionic antagonist, and may involve a direct action of DH beta E on nicotine receptor.
Binding of the nicotinic cholinergic antagonist, dihydro-beta-erythroidine, to rat brain tissue.[Pubmed:6502210]
J Neurosci. 1984 Dec;4(12):2906-11.
The nicotinic cholinergic antagonist, dihydro-beta-erythroidine, binds to two sites in rat cortical membranes with dissociation constants of 4 and 22 nM and respective apparent Bmax values of 52 and 164 fmol/mg of protein. Binding to the higher affinity site, defined by the use of 2 nM [3H]dihydro-beta-erythroidine, was saturable, reversible, and susceptible to protein denaturation. Binding was highest in the thalamus and lowest in the spinal cord and showed preferential enrichment in a synaptosomal subfraction of rat brain. Nicotine displaced [3H]dihydro-beta-erythroidine in a stereospecific manner, the (-)-isomer being approximately 6 times more potent than the (+)-isomer. The alkaloid nicotinic agonists, cytisine and lobeline, were potent inhibitors of binding, while acetylcholine in the presence of the cholinesterase inhibitor di-isopropylfluorophosphate was equipotent with (+)-nicotine. Binding was also inhibited by the muscarinic ligands, arecoline, atropine, and oxotremorine. The nicotinic antagonists mecamylamine, hexamethonium, and pempidine were essentially inactive in displacing [3H]dihydro-beta-erythroidine. These findings indicate that dihydro-beta-erythroidine binds to a nicotinic recognition site in rat brain which is neuromuscular, rather than ganglionic, in nature and that such binding is similar in several respects to that seen with nicotinic agonists. Whether such binding is to a nicotinic, as opposed to nicotinic cholinergic, recognition site or to a "common" nicotinic/muscarinic site is an issue that requires further study.


