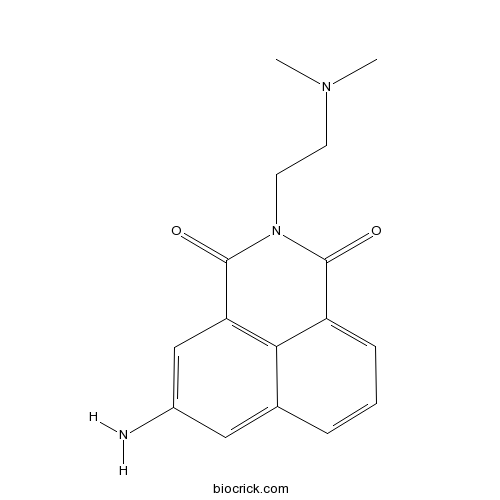
AmonafideDNA intercalator,Topo II inhibitor CAS# 69408-81-7 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 69408-81-7 | SDF | Download SDF |
| PubChem ID | 50515 | Appearance | Powder |
| Formula | C16H17N3O2 | M.Wt | 283.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AS1413 | ||
| Solubility | DMSO : 75 mg/mL (264.71 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 5-amino-2-[2-(dimethylamino)ethyl]benzo[de]isoquinoline-1,3-dione | ||
| SMILES | CN(C)CCN1C(=O)C2=CC=CC3=CC(=CC(=C32)C1=O)N | ||
| Standard InChIKey | UPALIKSFLSVKIS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H17N3O2/c1-18(2)6-7-19-15(20)12-5-3-4-10-8-11(17)9-13(14(10)12)16(19)21/h3-5,8-9H,6-7,17H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Amonafide is a topoisomerase II inhibitor and DNA intercalator that induces apoptotic signaling by blocking the binding of Topo II to DNA.In Vitro:Amonafide is a topoisomerase II inhibitor and DNA intercalator that induces apoptotic signaling by blocking the binding of Topo II to DNA[1]. Amonafide produces protein-associated DNA cleavage, single-strand breaks (SSB) and DPC and DNA double-strand cleavage. Amonafide (Nafidimide, 400 nM-2.4 μM) reduces the colony-forming ability of the leukemic cell lines in a dose-dependent manner[2]. Amonafide (0.05-0.4 μg/mL) reduces several tumor growth. However, Amonafide is active against only 12% of tumors compared with standard agents (5-fluorouracil, mitomycin C, cisplatin, and etoposide), which are active against more than 40% of tumors in the human bone marrow inhibitory range[3]. Amonafide inhibits the growth of HT-29, HeLa, and PC-3 cell lines, with IC50s of 4.67, 2.73, and 6.38 μM[4]. References: | |||||

Amonafide Dilution Calculator

Amonafide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5295 mL | 17.6473 mL | 35.2945 mL | 70.5891 mL | 88.2363 mL |
| 5 mM | 0.7059 mL | 3.5295 mL | 7.0589 mL | 14.1178 mL | 17.6473 mL |
| 10 mM | 0.3529 mL | 1.7647 mL | 3.5295 mL | 7.0589 mL | 8.8236 mL |
| 50 mM | 0.0706 mL | 0.3529 mL | 0.7059 mL | 1.4118 mL | 1.7647 mL |
| 100 mM | 0.0353 mL | 0.1765 mL | 0.3529 mL | 0.7059 mL | 0.8824 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 4.67, 2.73, and 6.38 for HT-29, HeLa, and PC3 cells, respectively
Amonafide is a novel topoisomerase II inhibitor. Topoisomerase II plays critical roles including DNA transcription, replication and chromosome segregation. Though the biological functions of topoisomerase II are important for insuring genomic integrity, the ability to interfere with topoisomerase II and generate enzyme mediated DNA damage is an effective strategy for cancer chemotherapy.
In vitro: Amonafide intercalated with DNA and disrupted the loading of topoisomerases. In contrast to the classic agents, amonafide was found to induce higher molecular weight fragmentation, resulting in the apoptosis without DNA cleavage. Amonafide was found to act in an ATP-independent manner and seemed unlikely to induce the chromosome translocations associated with treatmentinduced leukemia [1].
In vivo: Amonafide was found to be able to inhibit IP L1210 leukemia, with optimal increased life spans (ILS) of 61% to 106% following single 16 mg/kg dosing on days 1 to 9. Similar efficacy was noted against IP P388 murine leukemia and the SC implanted L1210 leukemia. Additionally, amonafide demonstrated activity against two nonleukemic IP implanted murine tumors, the M5076 sarcoma and the B16 melanoma [2].
Clinical trial: A multicenter, open-label, combination Phase II study was conducted for patients with sAML, evaluating the efficacy of cytarabine and amonafide administered as a continuous iv. Infusion of cytarabine on days 1-7 combined with 600 mg/m2/day for days 1-5[1].
References:
[1] Freeman CL,Swords R,Giles FJ. Amonafide: a future in treatment of resistant and secondary acute myeloid leukemia Expert Rev Hematol.2012 Feb;5(1):17-26.
[2] Saez R,Craig JB,Kuhn JG,Weiss GR,Koeller J,Phillips J,Havlin K,Harman G,Hardy J,Melink TJ, et al. Phase I clinical investigation of amonafide. J Clin Oncol.1989 Sep;7(9):1351-8.
- Lappaol H
Catalog No.:BCN8415
CAS No.:69394-18-9
- Sulbactam sodium
Catalog No.:BCC4852
CAS No.:69388-84-7
- Schisanhenol
Catalog No.:BCN2508
CAS No.:69363-14-0
- Pendulone
Catalog No.:BCN8248
CAS No.:69359-09-7
- Boc-D-Thr(Bzl)-OH
Catalog No.:BCC3454
CAS No.:69355-99-3
- Galanthamine hydrochloride
Catalog No.:BCC8277
CAS No.:5072-47-9
- Obtucarbamate A
Catalog No.:BCN3936
CAS No.:6935-99-5
- 2-Benzylaminopyridine
Catalog No.:BCC8565
CAS No.:6935-27-9
- CPPHA
Catalog No.:BCC1501
CAS No.:693288-97-0
- CYC116
Catalog No.:BCC2181
CAS No.:693228-63-6
- H-Ile-OtBu.HCl
Catalog No.:BCC2965
CAS No.:69320-89-4
- (R)-baclofen
Catalog No.:BCC4503
CAS No.:69308-37-8
- SB505124
Catalog No.:BCC5087
CAS No.:694433-59-5
- 17-PA
Catalog No.:BCC7452
CAS No.:694438-95-4
- Parvifuran
Catalog No.:BCN7780
CAS No.:69470-93-5
- 2-Methyl-4-isobutyrylphloroglucinol
Catalog No.:BCN7176
CAS No.:69480-03-1
- Boc-D-His(Tos)-OH
Catalog No.:BCC3405
CAS No.:69541-68-0
- Thymopentin
Catalog No.:BCN8347
CAS No.:69558-55-0
- Octadecyl caffeate
Catalog No.:BCN6609
CAS No.:69573-60-0
- Tupichilignan A
Catalog No.:BCN4257
CAS No.:69586-96-5
- 4-Hydroxybenzylamine
Catalog No.:BCN1805
CAS No.:696-60-6
- CRT0044876
Catalog No.:BCC5109
CAS No.:6960-45-8
- Chamaejasmine
Catalog No.:BCN3132
CAS No.:69618-96-8
- H-D-Asp(OMe)-OMe.HCl
Catalog No.:BCC2897
CAS No.:69630-50-8
Induction of apoptosis and suppression of ERCC1 expression by the potent amonafide analogue 8-c in human colorectal carcinoma cells.[Pubmed:23426174]
Anticancer Drugs. 2013 Apr;24(4):355-65.
Previous studies have reported that 8-c [6-(2-(2-(dimethylamino)ethylamino)ethylamino)-2-octyl-1H-benzo[de]isoquinoline-1 ,3(2H)-dione], a novel Amonafide analogue, was generated as a new anticancer candidate. However, little is known about its activity in chemoresistant cells. In this study, the antitumor effects of 8-c on the multi-drug-resistant human colorectal carcinoma cancer cell lines HCT-116/L-OHP and HCT-8/VCR have been investigated for the first time. 8-c showed similar concentration-dependent inhibitory activities against multi-drug-resistant cells and corresponding parental cell lines by the MTT assay after 48 h of treatment. 8-c treatment resulted in the induction of apoptosis, as evidenced by fluorescent staining analysis, comet assay data, and the increase in the number of apoptotic cells as detected by flow cytometry. Western blot, qPCR, and siRNA techniques were used to elucidate the molecular mechanism. Our study suggested that the apoptotic effect of 8-c can be attributed to the upregulation of p53, caspase-3, and cleaved poly(ADP-ribose) polymerase (PARP) and the downregulation of Bcl-2. Furthermore, ERCC1 is essential for nucleotide excision repair. ERCC1 expression was correlated with sensitivity to chemotherapy in various colon cancer cell lines. It is intriguing that decreases in ERCC1 protein and mRNA levels were also observed in the HCT-116/L-OHP and HCT-8/VCR cells after exposure to 8-c. Further transient transfection of multi-drug-resistant cells with ERCC1 siRNA enhanced 8-c-induced cytotoxicity. In contrast, epidermal growth factor-induced increase in ERCC1 protein levels was shown to rescue cell viability upon 8-c treatment. These findings suggest that 8-c has a strong potential to be developed as a new antitumor agent for the treatment of multi-drug-resistant colon cancer cells, and is worthy of further studies.
Phase III open-label randomized study of cytarabine in combination with amonafide L-malate or daunorubicin as induction therapy for patients with secondary acute myeloid leukemia.[Pubmed:25732165]
J Clin Oncol. 2015 Apr 10;33(11):1252-7.
PURPOSE: Secondary acute myeloid leukemia (sAML), defined as AML arising after a prior myelodysplastic syndrome or after antineoplastic therapy, responds poorly to current therapies. It is often associated with adverse karyotypic abnormalities and overexpression of proteins that mediate drug resistance. We performed a phase III trial to determine whether induction therapy with cytarabine and Amonafide L-malate, a DNA intercalator and non-ATP-dependent topoisomerase II inhibitor that evades drug resistance mechanisms, yielded a superior complete remission rate than standard therapy with cytarabine and daunorubicin in sAML. PATIENTS AND METHODS: Patients with previously untreated sAML were randomly assigned at a one-to-one ratio to cytarabine 200 mg/m(2) continuous intravenous (IV) infusion once per day on days 1 to 7 plus either Amonafide 600 mg/m(2) IV over 4 hours on days 1 to 5 (A + C arm) or daunorubicin 45 mg/m(2) IV over 30 minutes once per day on days 1 to 3 (D + C arm). RESULTS: The complete remission (CR) rate was 46% (99 of 216 patients) in A + C arm and 45% (97 of 217 patients) in D + C arm (P = .81). The 30- and 60-day mortality rates were 19% and 28% in A + C arm and 13% and 21% in D + C arm, respectively. CONCLUSION: Induction treatment with A + C did not improve the CR rate compared with D + C in patients with sAML.
Synthesis and evaluation of novel amonafide-polyamine conjugates as anticancer agents.[Pubmed:27762101]
Chem Biol Drug Des. 2017 May;89(5):670-680.
With the aim of upregulating antitumor efficacy and downregulating adverse effects, the amino group in the three-position of Amonafide aromatic ring was modified by coupling with different amine/polyamine motifs via two linkers. Two series of naphthalimide derivatives were designed and synthesized and evaluated for their antitumor properties in vitro and in vivo. The preliminary in vitro trials revealed that compounds with urea as the linker were not active, and the presence of aspirin elevated the potency of 6k against tumor cells, wound healing, and the protein expression of cyclic D1 and MMP9. The in vivo trials on three H22 tumor transplant models demonstrated that the combination of 6k and aspirin markedly improved the efficacy in terms of inhibitive effect, pulmonary metastasis, and extension of the life span. More importantly, the combination of 6k and aspirin displayed the reduced side-effects compared to that of Amonafide.
Design, antiviral and cytostatic properties of isoxazolidine-containing amonafide analogues.[Pubmed:26001344]
Bioorg Med Chem. 2015 Jul 1;23(13):3135-46.
A novel series of 5-arylcarbamoyl- and 5-arylmethyl-2-methylisoxazolidin-3-yl-3-phosphonates have been synthesized via cycloaddition of N-methyl-C-(diethoxyphosphoryl)nitrone with N-substituted naphthalimide acrylamides and N-allylnaphthalimides. All cis- and trans-isoxazolidine phosphonates obtained herein were assessed for antiviral activity against a broad range of DNA and RNA viruses. Isoxazolidines trans-9d and trans-9f exhibited the highest activity (EC50=8.9muM) toward cytomegalovirus. Compounds cis- and trans-9d as well as cis- and trans-9f were found potent against HSV and Vaccinia viruses (EC50 in the 45-58muM range), whereas isoxazolidines 10a and 10d suppressed replication of Coxsackie B4 and Punta Toro viruses (EC50 in the 45-73muM range). Antiproliferative evaluation of all obtained isoxazolidines revealed the promising activity of cis-9b, cis-9d, trans-9d, cis-9e, trans-9e, cis-9f and trans-9f toward tested cancer cell lines with IC50 in the 1.1-19muM range.


