Octadecyl caffeateCAS# 69573-60-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 69573-60-0 | SDF | Download SDF |
| PubChem ID | 5320237 | Appearance | Powder |
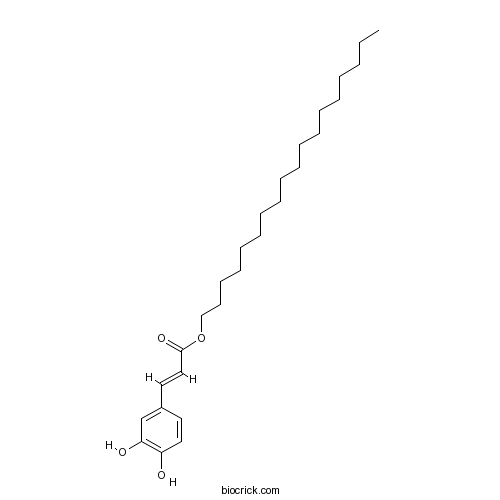
| Formula | C27H44O4 | M.Wt | 432.7 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | octadecyl (E)-3-(3,4-dihydroxyphenyl)prop-2-enoate | ||
| SMILES | CCCCCCCCCCCCCCCCCCOC(=O)C=CC1=CC(=C(C=C1)O)O | ||
| Standard InChIKey | QYVZEPLDLPYECM-XUTLUUPISA-N | ||
| Standard InChI | InChI=1S/C27H44O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-22-31-27(30)21-19-24-18-20-25(28)26(29)23-24/h18-21,23,28-29H,2-17,22H2,1H3/b21-19+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Octadecyl caffeate and coptisonine can stimulate glucose uptake at 25 and 50 ug/mL. 2. Octadecyl caffeate and octadecyl coumarate applied to the surface of susceptible varieties in laboratory bioassays reduced feeding and oviposition, as observed on roots of resistant varieties, and therefore are implicated in weevil resistance. 3. z-Octadecyl caffeate may have pain-relieving activity. |

Octadecyl caffeate Dilution Calculator

Octadecyl caffeate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3111 mL | 11.5554 mL | 23.1107 mL | 46.2214 mL | 57.7768 mL |
| 5 mM | 0.4622 mL | 2.3111 mL | 4.6221 mL | 9.2443 mL | 11.5554 mL |
| 10 mM | 0.2311 mL | 1.1555 mL | 2.3111 mL | 4.6221 mL | 5.7777 mL |
| 50 mM | 0.0462 mL | 0.2311 mL | 0.4622 mL | 0.9244 mL | 1.1555 mL |
| 100 mM | 0.0231 mL | 0.1156 mL | 0.2311 mL | 0.4622 mL | 0.5778 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Thymopentin
Catalog No.:BCN8347
CAS No.:69558-55-0
- Boc-D-His(Tos)-OH
Catalog No.:BCC3405
CAS No.:69541-68-0
- 2-Methyl-4-isobutyrylphloroglucinol
Catalog No.:BCN7176
CAS No.:69480-03-1
- Parvifuran
Catalog No.:BCN7780
CAS No.:69470-93-5
- 17-PA
Catalog No.:BCC7452
CAS No.:694438-95-4
- SB505124
Catalog No.:BCC5087
CAS No.:694433-59-5
- Amonafide
Catalog No.:BCC1249
CAS No.:69408-81-7
- Lappaol H
Catalog No.:BCN8415
CAS No.:69394-18-9
- Sulbactam sodium
Catalog No.:BCC4852
CAS No.:69388-84-7
- Schisanhenol
Catalog No.:BCN2508
CAS No.:69363-14-0
- Pendulone
Catalog No.:BCN8248
CAS No.:69359-09-7
- Boc-D-Thr(Bzl)-OH
Catalog No.:BCC3454
CAS No.:69355-99-3
- Tupichilignan A
Catalog No.:BCN4257
CAS No.:69586-96-5
- 4-Hydroxybenzylamine
Catalog No.:BCN1805
CAS No.:696-60-6
- CRT0044876
Catalog No.:BCC5109
CAS No.:6960-45-8
- Chamaejasmine
Catalog No.:BCN3132
CAS No.:69618-96-8
- H-D-Asp(OMe)-OMe.HCl
Catalog No.:BCC2897
CAS No.:69630-50-8
- H-D-Met-OMe.HCl
Catalog No.:BCC2998
CAS No.:69630-60-0
- Pd-C-II
Catalog No.:BCN4599
CAS No.:
- NSC 66811
Catalog No.:BCC2255
CAS No.:6964-62-1
- 5'-Methoxynobiletin
Catalog No.:BCN8031
CAS No.:6965-36-2
- Hesperetin 5-O-glucoside
Catalog No.:BCN3934
CAS No.:69651-80-5
- Trichloro-1,4-dimethoxybenzene
Catalog No.:BCN3494
CAS No.:69653-71-0
- Didanosine
Catalog No.:BCC3763
CAS No.:69655-05-6
Alkaloids from Coptis chinensis root promote glucose uptake in C2C12 myotubes.[Pubmed:24444890]
Fitoterapia. 2014 Mar;93:239-44.
The root of Coptis chinensis Franch. (COCH) is regularly used for medicinal purposes, and has been prescribed alone or in combination with other traditional herbs for the treatment of diabetes. To investigate the effects of COCH on glucose utilization by skeletal muscles, we prepared an ethanol extract of COCH root (COCH-Et) partitioned with dichloromethane, n-butanol, and water and tested its effects on glucose uptake in differentiated C2C12 myotubes. We found that dichloromethane and n-butanol sub-fractions of COCH-Et promoted glucose uptake in differentiated C2C12 cells at 50 mug/mL. Further fractionation of these preparations by using column chromatography, analysis of their effects on glucose uptake and characterization using nuclear magnetic resonance, mass spectrometry, and thin layer chromatography helped identify two new alkaloids, 8,13-dioxocoptisine hydroxide (1) and coptisonine (2), together with eleven known compounds. These were isolated from the dichloromethane layer of COCH-Et. In particular, exposure of C2C12 cells to berberine (6) at 12.5 and 6.25 mug/mL for 24h resulted in significant promotion of glucose uptake. Coptisonine (2) and Octadecyl caffeate (9) also stimulated glucose uptake at 25 and 50 mug/mL. These findings indicate that active constituents of COCH root may help alleviate hyperglycemia in diabetes by promoting glucose uptake by skeletal muscles.
[Study of chemical constituents in stem rind of Daphne giraldii].[Pubmed:16780156]
Zhongguo Zhong Yao Za Zhi. 2006 Apr;31(7):555-7.
OBJECTIVE: To study the constituents with the pain-relieving activity from the stem rind of Daphne giraldii. METHOD: The partition of the ethanol extract and chromatographic separation of the fractions were carried out by the monitoring of anelgesic pharmacological activity. The structures of the isolated compounds were determined by MS and NMR. RESULT: Four compounds were isolated from the pain-relieving fraction. Three of them were identified as diterpenes, gniditrin (1), gnidicin (2) and daphnetoxin (3). Compound 4 was determined as Z-Octadecyl caffeate. CONCLUSION: Compounds 1, 2 and 4 were isolated from the plant for the first time.
Resistance to the weevils Cylas puncticollis and Cylas brunneus conferred by sweetpotato root surface compounds.[Pubmed:23906084]
J Agric Food Chem. 2013 Aug 28;61(34):8141-7.
Seven resistant varieties of sweetpotato were compared with three susceptible varieties in field trials and laboratory bioassays and showed that resistance was an active process rather than an escape mechanism, as field resistant varieties also had reduced root damage and oviposition compared with susceptible varieties in the laboratory. Liquid chromatography-mass spectrometry (LC-MS) of root surface and epidermal extracts showed significant variation in the concentration of hexadecyl, heptadecyl, octadecyl, and quinic acid esters of caffeic and coumaric acid, with higher concentrations correlated with resistance. All compounds were synthesized to enable their positive identification. Octadecyl coumarate and Octadecyl caffeate applied to the surface of susceptible varieties in laboratory bioassays reduced feeding and oviposition, as observed on roots of resistant varieties, and therefore are implicated in weevil resistance. Segregating populations from breeding programs can use these compounds to identify trait loci for resistance and enable the development of resistant varieties.


