MisoprostolCytoprotective PGE1 analog CAS# 59122-46-2 |
- Guanfacine hydrochloride
Catalog No.:BCC1609
CAS No.:29110-48-3
- (R,R)-Formoterol
Catalog No.:BCC1293
CAS No.:67346-49-0
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
- Medetomidine
Catalog No.:BCC1736
CAS No.:86347-14-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 59122-46-2 | SDF | Download SDF |
| PubChem ID | 5282381 | Appearance | Powder |
| Formula | C22H38O5 | M.Wt | 382.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in ethanol and to 100 mM in DMSO | ||
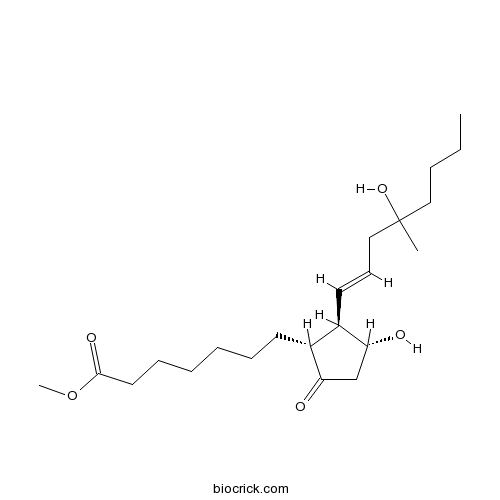
| Chemical Name | methyl 7-[(1R,2R,3R)-3-hydroxy-2-[(E)-4-hydroxy-4-methyloct-1-enyl]-5-oxocyclopentyl]heptanoate | ||
| SMILES | CCCCC(C)(CC=CC1C(CC(=O)C1CCCCCCC(=O)OC)O)O | ||
| Standard InChIKey | OJLOPKGSLYJEMD-URPKTTJQSA-N | ||
| Standard InChI | InChI=1S/C22H38O5/c1-4-5-14-22(2,26)15-10-12-18-17(19(23)16-20(18)24)11-8-6-7-9-13-21(25)27-3/h10,12,17-18,20,24,26H,4-9,11,13-16H2,1-3H3/b12-10+/t17-,18-,20-,22?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cytoprotective prostaglandin E1 analog that displays agonist activity at EP receptors. Ki values are 120, 250, 67 and 67 nM at cloned mouse EP1, EP2, EP3 and EP4 receptors respectively. Prevents NSAID-induced gastric ulceration. |

Misoprostol Dilution Calculator

Misoprostol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6142 mL | 13.0709 mL | 26.1417 mL | 52.2835 mL | 65.3544 mL |
| 5 mM | 0.5228 mL | 2.6142 mL | 5.2283 mL | 10.4567 mL | 13.0709 mL |
| 10 mM | 0.2614 mL | 1.3071 mL | 2.6142 mL | 5.2283 mL | 6.5354 mL |
| 50 mM | 0.0523 mL | 0.2614 mL | 0.5228 mL | 1.0457 mL | 1.3071 mL |
| 100 mM | 0.0261 mL | 0.1307 mL | 0.2614 mL | 0.5228 mL | 0.6535 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Alpha-Angelica lactone
Catalog No.:BCN5001
CAS No.:591-12-8
- (+)-Rhododendrol
Catalog No.:BCN7091
CAS No.:59092-94-3
- Albaspidin AP
Catalog No.:BCN2398
CAS No.:59092-91-0
- Dehydrotoxicarol
Catalog No.:BCN3991
CAS No.:59086-93-0
- Atropine sulfate monohydrate
Catalog No.:BCC3728
CAS No.:5908-99-6
- 8-Hydroxyhyperforin 8,1-hemiacetal
Catalog No.:BCN4091
CAS No.:59014-02-7
- alpha-Endorphin
Catalog No.:BCC1010
CAS No.:59004-96-5
- Bethanechol chloride
Catalog No.:BCC4566
CAS No.:590-63-6
- Betaine hydrochloride
Catalog No.:BCN6304
CAS No.:590-46-5
- Tolazoline HCl
Catalog No.:BCC4321
CAS No.:59-97-2
- Levodopa
Catalog No.:BCN1098
CAS No.:59-92-7
- Nitrofurazone
Catalog No.:BCC3825
CAS No.:59-87-0
- Neoisoliquiritin
Catalog No.:BCN2936
CAS No.:59122-93-9
- PSB 10 hydrochloride
Catalog No.:BCC7238
CAS No.:591771-91-4
- Sulforaphene
Catalog No.:BCN8179
CAS No.:592-95-0
- beta-Dihydroplumericinic acid
Catalog No.:BCN4092
CAS No.:59204-61-4
- 8(14),15-Isopimaradiene-3,18-diol
Catalog No.:BCN4093
CAS No.:59219-64-6
- Darutoside
Catalog No.:BCN4094
CAS No.:59219-65-7
- Erigeroside
Catalog No.:BCC8171
CAS No.:59219-76-0
- Laurocapram
Catalog No.:BCN8308
CAS No.:59227-89-3
- Chikusetsu Saponin Ib
Catalog No.:BCC8308
CAS No.:59252-87-8
- Rigosertib
Catalog No.:BCC4296
CAS No.:592542-59-1
- Rigosertib sodium
Catalog No.:BCC4067
CAS No.:592542-60-4
- Meptazinol HCl
Catalog No.:BCC4920
CAS No.:59263-76-2
Can the Induction of Labor with Misoprostol Increase Maternal Blood Loss?[Pubmed:28320031]
Rev Bras Ginecol Obstet. 2017 Feb;39(2):53-59.
Purpose To evaluate blood loss during Misoprostol-induced vaginal births and during cesarean sections after attempted Misoprostol induction. Methods We conducted a prospective observational study in 101 pregnant women indicated for labor induction; pre- and postpartum hemoglobin levels were measured to estimate blood loss during delivery. Labor was induced by administering 25 microg vaginal Misoprostol every 6 hours (with a maximum of 6 doses). The control group included 30 patients who spontaneously entered labor, and 30 patients who underwent elective cesarean section. Pre- and postpartum hemoglobin levels were evaluated using the analysis of variance for repeated measurements, showing the effects of time (pre- and postpartum) and of the group (with and without Misoprostol administration). Results There were significant differences between pre- and postpartum hemoglobin levels (p < 0.0001) with regard to Misoprostol-induced vaginal deliveries (1.6 +/- 1.4 mg/dL), non-induced vaginal deliveries (1.4 +/- 1.0 mg/dL), cesarean sections after attempted Misoprostol induction (1.5 +/- 1.0 mg/dL), and elective cesarean deliveries (1.8 +/- 1.1 mg/dL). However, the differences were proportional between the groups with and without Misoprostol administration, for both cesarean (p = 0.6845) and vaginal deliveries (p = 0.2694). Conclusions Labor induction using Misoprostol did not affect blood loss during delivery.
Community-based misoprostol for the prevention of post-partum haemorrhage: A narrative review of the evidence base, challenges and scale-up.[Pubmed:28357885]
Glob Public Health. 2018 Aug;13(8):1081-1097.
Achieving Sustainable Development Goal targets for 2030 will require persistent investment and creativity in improving access to quality health services, including skilled attendance at birth and access to emergency obstetric care. Community-based Misoprostol has been extensively studied and recently endorsed by the WHO for the prevention of post-partum haemorrhage. There remains little consolidated information about experience with implementation and scale-up to date. This narrative review of the literature aimed to identify the political processes leading to WHO endorsement of Misoprostol for the prevention of post-partum haemorrhage and describe ongoing challenges to the uptake and scale-up at both policy and community levels. We review the peer-reviewed and grey literature on expansion and scale-up and present the issues central to moving forward.
Misoprostol use for second-trimester termination of pregnancy among women with one or more previous cesarean deliveries.[Pubmed:28378361]
Int J Gynaecol Obstet. 2017 Jul;138(1):23-27.
OBJECTIVE: To establish the safety and efficacy of Misoprostol for second-trimester termination of pregnancy among women with one or more previous cesarean deliveries. METHODS: In a retrospective study, data were reviewed from women attending a reproductive health clinic in Bloemfontein, South Africa, for second-trimester termination between 2010 and 2013. The study group, comprising women with one or more previous cesareans, was compared with a control group, comprising women with no previous cesarean or uterine scarring. Procedure-specific information was compared, including Misoprostol use, termination duration, need for other methods (e.g. oxytocin), placenta delivery, termination outcome, and bleeding. RESULTS: The study group comprised 268 women: 231 (86.2%) with one and 37 (13.8%) with two previous cesareans. The control group comprised 266 women. Incomplete abortion was recorded in 223 (85.4%) of 261 women in the study group and 213 (80.4%) of 265 in the control group. The number of women with retained placenta was higher in the study than in the control group (158/261 [60.5%] vs 146/265 [55.1%]; P<0.001). Severe bleeding was observed only in the control group (7/266 [2.6%]). No uterine rupture was observed. CONCLUSION: Misoprostol was safe for second-trimester termination among women with previous cesareans; however, the efficacy of the local regimen was reduced owing to high placental retention.
Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells.[Pubmed:9313928]
Br J Pharmacol. 1997 Sep;122(2):217-24.
1. Eight types and subtypes of the mouse prostanoid receptor, the prostaglandin D (DP) receptor, the prostaglandin F (FP) receptor, the prostaglandin I (IP) receptor, the thromboxane A (TP) receptor and the EP1, EP2, EP3 and EP4 subtypes of the prostaglandin E receptor, were stably expressed in Chinese hamster ovary cells. Their ligand binding characteristics were examined with thirty two prostanoids and their analogues by determining the Ki values from the displacement curves of radioligand binding to the respective receptors. 2. The DP, IP and TP receptors showed high ligand binding specificity and only bound their own putative ligands with high affinity such as PGD2, BW245C and BW868C for DP, cicaprost, iloprost and isocabacyclin for IP, and S-145, I-BOP and GR 32191 for TP. 3. The FP receptor bound PGF2 alpha and fluprostenol with Ki values of 3-4 nM. In addition, PGD2, 17-phenyl-PGE2, STA2, I-BOP, PGE2 and M&B-28767 bound to this receptor with Ki values less than 100 nM. 4. The EP1 receptor bound 17-phenyl-PGE2, sulprostone and iloprost in addition to PGE2 and PGE1, with Ki values of 14-36 nM. 16,16-dimethyl-PGE2 and two putative EP1 antagonists, AH6809 and SC-19220, did not show any significant binding to this receptor. M&B-28767, a putative EP3 agonist, and Misoprostol, a putative EP2/EP3 agonist, also bound to this receptor with Ki values of 120 nM. 5. The EP2 and EP4 receptors showed similar binding profiles. They bound 16,16-dimethyl PGE2 and 11-deoxy-PGE1 in addition to PGE2 and PGE1. The two receptors were discriminated by butaprost, AH-13205 and AH-6809 that bound to the EP2 receptor but not to the EP4 receptor, and by 1-OH-PGE1 that bound to the EP4 but not to the EP2 receptor. 6. The EP3 receptor showed the broadest binding profile, and bound sulprostone, M&B-28767, GR63799X, 11-deoxy-PGE1, 16,16-dimethyl-PGE2 and 17-phenyl-PGE2, in addition to PGE2 and PGE1, with Ki values of 0.6-3.7 nM. In addition, three IP ligands, iloprost, carbacyclin and isocarbacyclin, and one TP ligand, STA2, bound to this receptor with Ki values comparable to the Ki values of these compounds for the IP and TP receptors, respectively. 7. 8-Epi-PGF2 alpha showed only weak binding to the IP, TP, FP, EP2 and EP3 receptor at 10 microM concentration.
Characterization of dilator prostanoid receptors in the fetal rabbit ductus arteriosus.[Pubmed:7965740]
J Pharmacol Exp Ther. 1994 Oct;271(1):390-6.
The purpose of this study was to determine which receptors mediate the dilator effects of prostaglandins (PGs) on the ductus arteriosus of the fetal rabbit. Isolated rings of the vessel from fetal New Zealand White rabbits were precontracted with indomethacin (1 microM) and potassium (25 mM) in 100 to 110 mmHg oxygen, and the dilator effects of a range of synthetic prostanoids were quantified by cumulative relaxant concentration-effect curves. The potencies of agonists were quantified with reference to PGE2 by the equieffective molar ratio (EMR): EC50 test agonist/EC50 PGE2. The effects on these responses of available antagonists were also studied. None of a range of synthetic prostanoids with selective agonism of EP1, EP2 or EP3 receptors was as potent as PGE2. The rank order of potency was as follows: PGE2 (EC50 = 0.36 nM [95% confidence intervals [CI] = 0.32-0.41, n = 44], EMR = unity) >> Misoprostol (EMR 145, 95% CI 73-217) > [1R-[1 alpha (Z),2 beta (R*),3 alpha]]-4-(benzoyl-amino)phenyl 7-[3 hydroxy-2(2-hydroxy-3-phenoxypropoxy)-5- oxocyclopentyl]-4-heptenoate, single enantiomer (GR 63799K) (EMR 685, 95% CI 427-944) >> ((+/-)-trans-2-[4[(1-hydroxphenyl) phenyl]-5-oxocylopentaneheptanoic acid (AH13205) (EMR > 100,000) > or = sulprostone (EMR > 10,000) > or = 0. The EP1 antagonists, 6-isopropoxy-9-oxoxanthine-2-carboxylic acid (AH6809) (10 microM) and 8-clorodi-benz[b,f][1,4]oxazepine-10 (11H)-carboxylic acid, 2-acetylhydrazide (SC19220) (30 microM), had no significant effect on the sensitivity of the ductus to PGE2.(ABSTRACT TRUNCATED AT 250 WORDS)
Prevention of NSAID-induced gastric ulcer with misoprostol: multicentre, double-blind, placebo-controlled trial.[Pubmed:2904006]
Lancet. 1988 Dec 3;2(8623):1277-80.
A double-blind, placebo-controlled study was carried out to see whether the synthetic E prostaglandin, Misoprostol, would prevent gastric ulcer induced by non-steroidal anti-inflammatory drugs (NSAIDs). 420 patients with osteoarthritis and NSAID-associated abdominal pain were studied; they were receiving ibuprofen, piroxicam, or naproxen. Endoscopy was done at entry and after 1, 2, and 3 months of continuous treatment with 100 micrograms or 200 micrograms Misoprostol or placebo, given four times daily with meals and at bedtime, concurrently with the NSAID. Abdominal pain was rated independently by patients and physicians. A treatment failure was defined as development of a gastric ulcer. Gastric ulcers (0.3 cm in diameter or greater) occurred less frequently (p less than 0.001) in both Misoprostol treatment groups (5.6% 100 micrograms and 1.4% 200 micrograms) than in the placebo group (21.7%). The significant difference in ulcer formation between the placebo and the Misoprostol treatment groups remained when comparisons were restricted to ulcers greater than 0.5 cm in diameter (12.3% placebo, 4.2% 100 micrograms Misoprostol, and 0.7% 200 micrograms Misoprostol). Mild to moderate, self-limiting diarrhoea was the most frequently reported adverse effect attributed to Misoprostol. These results provide the first clear indication that NSAID-induced ulcers are preventable.


