PSB 10 hydrochloridePotent, highly selective hA3 receptor antagonist/inverse agonist CAS# 591771-91-4 |
- PSI
Catalog No.:BCC1124
CAS No.:158442-41-2
- Salinosporamide A (NPI-0052, Marizomib)
Catalog No.:BCC2094
CAS No.:437742-34-2
- Aclacinomycin A
Catalog No.:BCC1232
CAS No.:57576-44-0
- E-64
Catalog No.:BCC1222
CAS No.:66701-25-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 591771-91-4 | SDF | Download SDF |
| PubChem ID | 76453660 | Appearance | Powder |
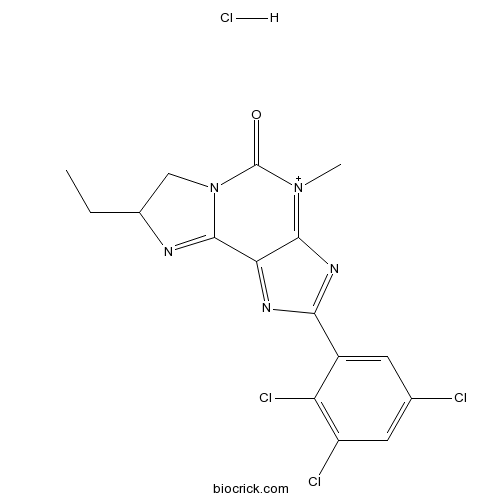
| Formula | C16H14Cl4N5O+ | M.Wt | 434.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in DMSO and to 10 mM in ethanol | ||
| Chemical Name | 8-ethyl-4-methyl-2-(2,3,5-trichlorophenyl)-7,8-dihydroimidazo[2,1-f]purin-4-ium-5-one;hydrochloride | ||
| SMILES | CCC1CN2C(=N1)C3=NC(=NC3=[N+](C2=O)C)C4=CC(=CC(=C4Cl)Cl)Cl.Cl | ||
| Standard InChIKey | FKYABZZYLDPWRN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H13Cl3N5O.ClH/c1-3-8-6-24-15(20-8)12-14(23(2)16(24)25)22-13(21-12)9-4-7(17)5-10(18)11(9)19;/h4-5,8H,3,6H2,1-2H3;1H/q+1; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and highly selective antagonist for the human adenosine A3 receptor, with low affinity for the rat A3 receptor (Ki values are 0.44 and > 17000 nM respectively). Displays > 3800-fold selectivity over human A1, A2A and A2B receptors (Ki values are 4.1, 3.3 and 30 μM respectively) and > 1800-fold selectivity over rat A1 and A2A receptors. Acts as an inverse agonist in the [35S]GTPγS binding assay in hA3-CHO cells (IC50 = 4 nM). Produces thermal hyperalgesia in mice in vivo. |

PSB 10 hydrochloride Dilution Calculator

PSB 10 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3036 mL | 11.5181 mL | 23.0362 mL | 46.0723 mL | 57.5904 mL |
| 5 mM | 0.4607 mL | 2.3036 mL | 4.6072 mL | 9.2145 mL | 11.5181 mL |
| 10 mM | 0.2304 mL | 1.1518 mL | 2.3036 mL | 4.6072 mL | 5.759 mL |
| 50 mM | 0.0461 mL | 0.2304 mL | 0.4607 mL | 0.9214 mL | 1.1518 mL |
| 100 mM | 0.023 mL | 0.1152 mL | 0.2304 mL | 0.4607 mL | 0.5759 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Neoisoliquiritin
Catalog No.:BCN2936
CAS No.:59122-93-9
- Misoprostol
Catalog No.:BCC5240
CAS No.:59122-46-2
- Alpha-Angelica lactone
Catalog No.:BCN5001
CAS No.:591-12-8
- (+)-Rhododendrol
Catalog No.:BCN7091
CAS No.:59092-94-3
- Albaspidin AP
Catalog No.:BCN2398
CAS No.:59092-91-0
- Dehydrotoxicarol
Catalog No.:BCN3991
CAS No.:59086-93-0
- Atropine sulfate monohydrate
Catalog No.:BCC3728
CAS No.:5908-99-6
- 8-Hydroxyhyperforin 8,1-hemiacetal
Catalog No.:BCN4091
CAS No.:59014-02-7
- alpha-Endorphin
Catalog No.:BCC1010
CAS No.:59004-96-5
- Bethanechol chloride
Catalog No.:BCC4566
CAS No.:590-63-6
- Betaine hydrochloride
Catalog No.:BCN6304
CAS No.:590-46-5
- Tolazoline HCl
Catalog No.:BCC4321
CAS No.:59-97-2
- Sulforaphene
Catalog No.:BCN8179
CAS No.:592-95-0
- beta-Dihydroplumericinic acid
Catalog No.:BCN4092
CAS No.:59204-61-4
- 8(14),15-Isopimaradiene-3,18-diol
Catalog No.:BCN4093
CAS No.:59219-64-6
- Darutoside
Catalog No.:BCN4094
CAS No.:59219-65-7
- Erigeroside
Catalog No.:BCC8171
CAS No.:59219-76-0
- Laurocapram
Catalog No.:BCN8308
CAS No.:59227-89-3
- Chikusetsu Saponin Ib
Catalog No.:BCC8308
CAS No.:59252-87-8
- Rigosertib
Catalog No.:BCC4296
CAS No.:592542-59-1
- Rigosertib sodium
Catalog No.:BCC4067
CAS No.:592542-60-4
- Meptazinol HCl
Catalog No.:BCC4920
CAS No.:59263-76-2
- Acyclovir
Catalog No.:BCC3929
CAS No.:59277-89-3
- Decursin
Catalog No.:BCN5335
CAS No.:5928-25-6
Antinociceptive effects of novel A2B adenosine receptor antagonists.[Pubmed:14563788]
J Pharmacol Exp Ther. 2004 Jan;308(1):358-66.
Caffeine, an adenosine A1, A2A, and A2B receptor antagonist, is frequently used as an adjuvant analgesic in combination with nonsteroidal anti-inflammatory drugs or opioids. In this study, we have examined the effects of novel specific adenosine receptor antagonists in an acute animal model of nociception. Several A2B-selective compounds showed antinociceptive effects in the hot-plate test. In contrast, A1- and A2A-selective compounds did not alter pain thresholds, and an A3 adenosine receptor antagonist produced thermal hyperalgesia. Evaluation of psychostimulant effects of these compounds in the open field showed only small effects of some antagonists at high doses. Coadministration of low, subeffective doses of A2B-selective antagonists with a low dose of morphine enhanced the efficacy of morphine. Our results indicate that analgesic effects of caffeine are mediated, at least in part, by A2B adenosine receptors.
Medicinal chemistry of adenosine A3 receptor ligands.[Pubmed:12570761]
Curr Top Med Chem. 2003;3(4):445-62.
A(3) Adenosine receptors (ARs) exhibit large species differences. Potent, selective agonists for rat (e.g. Cl-IB-MECA, 5) and human A(3) ARs (e.g. PENECA, 17, and analogs) have been developed during the past years. Potent, selective antagonists for human A(3) ARs include the imidazopurinones PSB-10 (28) and PSB-11 (29), the pyrazolotriazolopyrimidines MRE-3005F20 (38) and analogs, and the dihydropyridines (e.g. MRS-1334, 50). For rat A(3) ARs only moderately potent antagonists have been identified, such as the pyridine derivative MRS-1523 (51) and the flavonoid MRS-1067 (52), both of which exhibit only a low degree of selectivity versus the other AR subtypes. Selective antagonist radioligands for the human A(3) receptor, [(3)H]MRE-3008F20 and [(3)H]PSB-11, have been prepared, while A(3)-selective agonist radioligands are still lacking. Recent developments also include allosteric modulators, irreversibly binding antagonists, fluorescence-labelled agonists, partial agonists and inverse agonists for A(3)ARs. Site-directed mutagenesis and molecular modeling studies have been performed in order to obtain information about the ligand binding site and the process of receptor activation. A(3)Adenosine receptors have recently attracted considerable interest as novel drug targets. A(3) Agonists may have potential as cardioprotective and cerebroprotective agents, for the treatment of asthma, as antiinflammatory and immunosuppressive agents, and in cancer therapy as cytostatics and chemoprotective compounds. A(3) AR antagonists might be therapeutically useful for the acute treatment of stroke, for glaucoma, and also as antiasthmatic and antiallergic drugs, since A(3)receptors cannot only mediate antiinflammatory, but also proinflammatory responses. The future development of further pharmacological tools, including potent, selective antagonists for rat A(3) receptors and selective agonist radioligands for rat and human receptors will facilitate the evaluation of the (patho)physiological roles of A(3) receptors and the pharmacological potential of their ligands.
2-Phenylimidazo[2,1-i]purin-5-ones: structure-activity relationships and characterization of potent and selective inverse agonists at Human A3 adenosine receptors.[Pubmed:12517430]
Bioorg Med Chem. 2003 Feb 6;11(3):347-56.
Structure-activity relationships of 2-phenyl-imidazo[2,1-i]purin-5-ones as ligands for human A(3) adenosine receptors (ARs) were investigated. An ethyl group in the 8-position of the imidazoline ring of 4-methyl-2-phenyl-imidazopurinone leading to chiral compounds was found to increase affinity for human A(3) ARs by several thousand-fold. Propyl substitution instead of methyl at N4 decreased A(3) affinity but increased A(1) affinity leading to potent A(1)-selective AR antagonists. The most potent A(1) antagonist of the present series was (S)-8-ethyl-2-phenyl-4-propyl-4,5,7,8-tetrahydro-1H-imidazo[2,1-i]purin-5-one (S-3) exhibiting a K(i) value of 7.4 nM at rat A(1) ARs and greater than 100-fold selectivity versus rat A(2A) and human A(3) ARs. At human A(1) ARs 2-phenylimidazo[2,1-i]purin-5-ones were generally less potent and therefore less A(1)-selective (S-3: K(i)=98 nM). 2-, 3-, or 4-Mono-chlorination of the 2-phenyl ring reduced A(3) affinity but led to an increase in affinity for A(1) ARs, whereas di- (3,4-dichloro) or polychlorination (2,3,5-trichloro) increased A(3) affinity. The most potent and selective A(3) antagonist of the present series was the trichlorophenyl derivative (R)-8-ethyl-4-methyl-2-(2,3,5-trichlorophenyl)-4,5,7,8-tetrahydro-1H-imidazo[2,1- i]purin-5-one (R-8) exhibiting a subnanomolar K(i) value at human A(3) ARs and greater than 800-fold selectivity versus the other AR subtypes. Methylation of 4-alkyl-2-phenyl-substituted imidazo[2,1-i]purin-5-ones led exclusively to the N9-methyl derivatives, which exhibited largely reduced AR affinities as compared to the unmethylated compounds. [35S]GTP gamma S binding studies of the most potent 2-phenyl-imidazo[2,1-i]purin-5-ones at membranes of Chinese hamster ovary cells expressing the human A(3) AR revealed that the compounds were inverse agonists at A(3) receptors under standard test conditions. Due to their high A(3) affinity, selectivity, and relatively high water-solubility, 2-phenyl-imidazo[2,1-i]purin-5-ones may become useful research tools.


