Aclacinomycin ATopoisomerase I and II inhibitor CAS# 57576-44-0 |
- MLN2238
Catalog No.:BCC2092
CAS No.:1072833-77-2
- MG-132
Catalog No.:BCC1227
CAS No.:133407-82-6
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- Bortezomib (PS-341)
Catalog No.:BCC1238
CAS No.:179324-69-7
- Salinosporamide A (NPI-0052, Marizomib)
Catalog No.:BCC2094
CAS No.:437742-34-2
- Gliotoxin
Catalog No.:BCN3894
CAS No.:67-99-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 57576-44-0 | SDF | Download SDF |
| PubChem ID | 308141 | Appearance | Powder |
| Formula | C42H53NO15 | M.Wt | 811.87 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
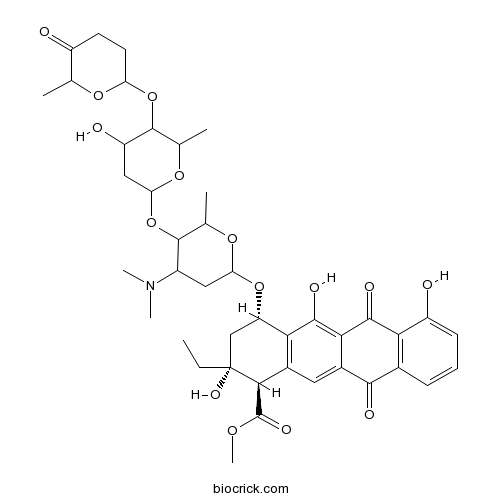
| Chemical Name | methyl (1R,2R,4S)-4-[4-(dimethylamino)-5-[4-hydroxy-6-methyl-5-(6-methyl-5-oxooxan-2-yl)oxyoxan-2-yl]oxy-6-methyloxan-2-yl]oxy-2-ethyl-2,5,7-trihydroxy-6,11-dioxo-3,4-dihydro-1H-tetracene-1-carboxylate | ||
| SMILES | CCC1(CC(C2=C(C1C(=O)OC)C=C3C(=C2O)C(=O)C4=C(C3=O)C=CC=C4O)OC5CC(C(C(O5)C)OC6CC(C(C(O6)C)OC7CCC(=O)C(O7)C)O)N(C)C)O | ||
| Standard InChIKey | USZYSDMBJDPRIF-XCKHQVGJSA-N | ||
| Standard InChI | InChI=1S/C42H53NO15/c1-8-42(51)17-28(33-22(35(42)41(50)52-7)14-23-34(38(33)49)37(48)32-21(36(23)47)10-9-11-26(32)45)56-30-15-24(43(5)6)39(19(3)54-30)58-31-16-27(46)40(20(4)55-31)57-29-13-12-25(44)18(2)53-29/h9-11,14,18-20,24,27-31,35,39-40,45-46,49,51H,8,12-13,15-17H2,1-7H3/t18?,19?,20?,24?,27?,28-,29?,30?,31?,35-,39?,40?,42+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Aclarubicin (aclacinomycin A), an anthracycline drug, specific inhibitor of the 20S proteasome chymotrypsin-like activity. | |||||
| Targets | 20S proteasome | |||||

Aclacinomycin A Dilution Calculator

Aclacinomycin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2317 mL | 6.1586 mL | 12.3172 mL | 24.6345 mL | 30.7931 mL |
| 5 mM | 0.2463 mL | 1.2317 mL | 2.4634 mL | 4.9269 mL | 6.1586 mL |
| 10 mM | 0.1232 mL | 0.6159 mL | 1.2317 mL | 2.4634 mL | 3.0793 mL |
| 50 mM | 0.0246 mL | 0.1232 mL | 0.2463 mL | 0.4927 mL | 0.6159 mL |
| 100 mM | 0.0123 mL | 0.0616 mL | 0.1232 mL | 0.2463 mL | 0.3079 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Aclacinomycin A is a dual inhibitor of topoisomerase I and II [1].
Aclacinomycin A is an anticancer drug which can reduce the tumor with minimal damage to normal cells. Aclacinomycin A shows potency against a wide variety of solid tumours and haematological malignancies. In A549, HepG2 and MCF-7 cells, Aclacinomycin A shows cytotoxic activity with IC50 values of 0.27μM, 0.32μM and 0.62μM, respectively. Aclacinomycin A induces cell apoptosis in these cells and the effects change to be necrosis when the incubation time is prolonged. Aclacinomycin A is demonstrated to increase the activity of both caspase-3 and caspase-8, thus inducing the activation of PARP. Apart from that, as an inhibitor of opoisomerases, Aclacinomycin A is found to induce DNA damage in V79 and irs-2 cells. Aclacinomycin A is used to treat acute leukaemias, lymphomas and other solid tumors through its inhibition of topo II [1, 2].
References:
[1] Hajji N, Mateos S, Pastor N, Domínguez I, Cortés F. Induction of genotoxic and cytotoxic damage by aclarubicin, a dual topoisomerase inhibitor. Mutat Res. 2005 May 2;583(1):26-35.
[2] Rogalska A, Szwed M, Jó?wiak Z. Aclarubicin-induced apoptosis and necrosis in cells derived from human solid tumours. Mutat Res. 2010 Jul 19;700(1-2):1-10.
- Norcepharadione B
Catalog No.:BCN5784
CAS No.:57576-41-7
- (2,4-Dihydroxyphenyl)acetonitrile
Catalog No.:BCN5783
CAS No.:57576-34-8
- (24S)-Cycloartane-3,24,25-triol 24,25-acetonide
Catalog No.:BCN1414
CAS No.:57576-31-5
- (3beta,24xi)-Cycloartane-3,24,25-triol
Catalog No.:BCN5782
CAS No.:57576-29-1
- PNU 37883 hydrochloride
Catalog No.:BCC7262
CAS No.:57568-80-6
- Isofuranodiene
Catalog No.:BCN5781
CAS No.:57566-47-9
- SNOG
Catalog No.:BCC6714
CAS No.:57564-91-7
- Notoginsenoside S
Catalog No.:BCN8371
CAS No.:575446-95-6
- H-His(Nτ-Me)-OMe.2HCl
Catalog No.:BCC2958
CAS No.:57519-09-2
- 2-Methylsulfanylpyrimidin-4(3H)-one
Catalog No.:BCC8582
CAS No.:5751-20-2
- 6-Benzyloxypurine
Catalog No.:BCC8770
CAS No.:57500-07-9
- 3-Benzalphthalide
Catalog No.:BCC8621
CAS No.:575-61-1
- Cycloartane-3,24,25-triol
Catalog No.:BCC8922
CAS No.:57586-98-8
- Biocytin
Catalog No.:BCC7659
CAS No.:576-19-2
- Piperenone
Catalog No.:BCN6578
CAS No.:57625-31-7
- Fenobam
Catalog No.:BCC7345
CAS No.:57653-26-6
- Baccatin IV
Catalog No.:BCN5785
CAS No.:57672-77-2
- 1-Dehydroxybaccatin IV
Catalog No.:BCN7211
CAS No.:57672-78-3
- Baccatin VI
Catalog No.:BCN7229
CAS No.:57672-79-4
- Palmitic acid-1-13C
Catalog No.:BCC8229
CAS No.:57677-53-9
- Kansuinine B
Catalog No.:BCN3766
CAS No.:57685-46-8
- Flavanomarein
Catalog No.:BCN6429
CAS No.:577-38-8
- 2-Acetylbenzoic acid
Catalog No.:BCN5786
CAS No.:577-56-0
- Kansuinine A
Catalog No.:BCN3765
CAS No.:57701-86-7
RGD-modified liposomes enhance efficiency of aclacinomycin A delivery: evaluation of their effect in lung cancer.[Pubmed:26316700]
Drug Des Devel Ther. 2015 Aug 11;9:4613-20.
In this study, long-circulating Arg-Gly-Asp (RGD)-modified Aclacinomycin A (ACM) liposomes were prepared by thin film hydration method. Their morphology, particle size, encapsulation efficiency, and in vitro release were investigated. The RGD-ACM liposomes was about 160 nm in size and had the visual appearance of a yellowish suspension. The zeta potential was -22.2 mV and the encapsulation efficiency was more than 93%. The drug-release behavior of the RGD-ACM liposomes showed a biphasic pattern, with an initial burst release and followed by sustained release at a constant rate. After being dissolved in phosphate-buffered saline (pH 7.4) and kept at 4 degrees C for one month, the liposomes did not aggregate and still had the appearance of a milky white colloidal solution. In a pharmacokinetic study, rats treated with RGD-ACM liposomes showed slightly higher plasma concentrations than those treated with ACM liposomes. Maximum plasma concentrations of RGD-ACM liposomes and ACM liposomes were 4,532 and 3,425 ng/mL, respectively. RGD-ACM liposomes had a higher AUC0-infinity (1.54-fold), mean residence time (2.09-fold), and elimination half-life (1.2-fold) when compared with ACM liposomes. In an in vivo study in mice, both types of liposomes inhibited growth of human lung adenocarcinoma (A549) cells and markedly decreased tumor size when compared with the control group. There were no obvious pathological tissue changes in any of the treatment groups. Our results indicate that RGD-modified ACM liposomes have a better antitumor effect in vivo than their unmodified counterparts.
Low-dose arsenic trioxide combined with aclacinomycin A synergistically enhances the cytotoxic effect on human acute myelogenous leukemia cell lines by induction of apoptosis.[Pubmed:25739941]
Leuk Lymphoma. 2015;56(11):3159-67.
Acute myeloid leukemia (AML) is a common disorder in the elderly. Although remarkable progress has been made over recent decades, the outcome remains poor. Thus, the development of a more effective method to overcome this problem is necessary. In this study, we aimed to investigate the synergistic cytotoxic effect of low-dose arsenic trioxide (As2O3) combined with Aclacinomycin A (ACM) on the human AML cell lines KG-1a and HL-60, and to clarify the underlying mechanism. Results showed that As2O3 combined with ACM exerted a synergistic cytotoxic effect by activation of the apoptosis pathway. Additionally, we found that the combination treatment decreased Bcl-2, c-IAP and XIAP expression but increased SMAC and caspase-3 expression more significantly than the single drug treatments. Furthermore, combination index (CI) values were < 1 in all matched combination groups. Additional evaluation of As2O3 combined with ACM as a potential therapeutic benefit for AML seems warranted.


