Bortezomib (PS-341)Proteasome Inhibitor CAS# 179324-69-7 |
- PSI
Catalog No.:BCC1124
CAS No.:158442-41-2
- Salinosporamide A (NPI-0052, Marizomib)
Catalog No.:BCC2094
CAS No.:437742-34-2
- Aclacinomycin A
Catalog No.:BCC1232
CAS No.:57576-44-0
- E-64
Catalog No.:BCC1222
CAS No.:66701-25-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 179324-69-7 | SDF | Download SDF |
| PubChem ID | 387447 | Appearance | Powder |
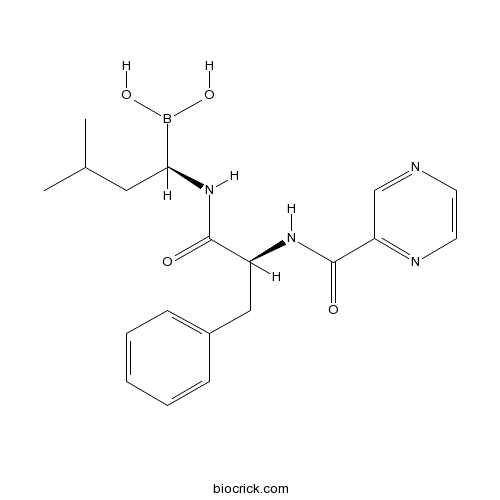
| Formula | C19H25BN4O4 | M.Wt | 384.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | PS-341; Brotezamide; DPBA; LDP 341; MG 341; Radiciol; NSC 681239 | ||
| Solubility | DMSO : ≥ 83.3 mg/mL (216.79 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [(1R)-3-methyl-1-[[(2S)-3-phenyl-2-(pyrazine-2-carbonylamino)propanoyl]amino]butyl]boronic acid | ||
| SMILES | B(C(CC(C)C)NC(=O)C(CC1=CC=CC=C1)NC(=O)C2=NC=CN=C2)(O)O | ||
| Standard InChIKey | GXJABQQUPOEUTA-RDJZCZTQSA-N | ||
| Standard InChI | InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bortezomib (PS-341) is a potent inhibitor of 20S proteasome with Ki of 0.6 nM. | |||||
| Targets | 20S proteasome | |||||
| IC50 | 0.6 nM (Ki) | |||||
| Cell experiment [1]: | |
| Cell lines | Canine malignant melanoma cell lines (CMM-1, CMM-2, ChMC, KMeC, LMeC, OMJ, OMS, OMK, and NML) |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 72h; IC50=3.5~5.6 nM (nine kinds of cells) |
| Applications | Bortezomib potently suppressed the growth in 21 drugs, while other compounds had no or minimal effect on cell growth. We thus focused on bortezomib and examined its growth inhibitory properties against nine canine malignant melanoma cell lines (CMM-1, CMM-2, ChMC, KMeC, LMeC, OMJ, OMS, OMK, and NML). Bortezomib inhibited the growth of all cell lines with calculated IC50 values of 3.5~5.6 nM. |
| Animal experiment [1]: | |
| Animal models | Nude athymic mice |
| Dosage form | 0.8 mg/kg; intravenous injection |
| Application | The in vivo growth inhibitory activity of bortezomib against CMM-1 cells was evaluated using a xenograft mouse model. Bortezomib significantly suppressed the growth of tumours after Day 4 of treatment (P < 0.01, control vs. bortezomib). Tumours from the bortezomib-treated mice showed a significant decrease in mitotic index compared to controls (P<0.01). Similarly, the Ki67 index was significantly decreased in tumours excised from the bortezomib-treated mice when compared to controls (P < 0.01). |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Ito K, Kobayashi M, Kuroki S, et al. The proteasome inhibitor bortezomib inhibits the growth of canine malignant melanoma cells in vitro and in vivo[J]. The Veterinary Journal, 2013, 198(3): 577-582. | |

Bortezomib (PS-341) Dilution Calculator

Bortezomib (PS-341) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6025 mL | 13.0127 mL | 26.0254 mL | 52.0508 mL | 65.0635 mL |
| 5 mM | 0.5205 mL | 2.6025 mL | 5.2051 mL | 10.4102 mL | 13.0127 mL |
| 10 mM | 0.2603 mL | 1.3013 mL | 2.6025 mL | 5.2051 mL | 6.5064 mL |
| 50 mM | 0.0521 mL | 0.2603 mL | 0.5205 mL | 1.041 mL | 1.3013 mL |
| 100 mM | 0.026 mL | 0.1301 mL | 0.2603 mL | 0.5205 mL | 0.6506 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
Bortezomib, a proteasome inhibitor with anti-pancreatic cancer activity, was assessed for its role in ceramide production.
Abstract
The resistance to bortezomib, a 20S proteasome inhibitor, in MM cell lines is correlated with mitochondrial activities and could be eliminated through regulation of mitochondrial genes.
Abstract
Bortezomib, a proteasome inhibitor with modes anti-malignant glioma activity, was reviewed in terms of its effects and disadvantages.
Abstract
Instead of synergistically killing multiple myeloma cells, bortezomib inhibited the replication and spread of VSV in vitro through suppressing NF-KB activation resulting in diminished anti-tumor activity and increased VSV response. However, the combination of those two drugs exhibited synergistic effects in vivo to reduce tumor burden in two syngeneic, immunocompetent myeloma models.
Abstract
The treatment of bortezomib combined with idarubicin was well tolerated in older AML patients with the maximum tolerated dose of 1.2 mg/m(2) and 10 mg/m(2) respectively.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bortezomib (originally codenamed PS-341) is the first therapeutic proteasome inhibitor to the tested in humans. It is approved in the U.S. for treating relapsed multiple myeloma and mantle cell lymphoma. [1] The drug is an N-protected dipeptide and can be written as Pyz-Phe-boroLeu, which stands for pyrazinoic acid, phenylalanine and Leucine with a boronic acid instead of a carboxylic acid. Peptides are written N-terminus to C-terminus, and this convention is used here even though the "C-terminus" is a boronic acid instead of a carboxylic acid. While multiple mechanisms are likely to be involved, proteasome inhibition may prevent degradation of pro-apoptotic factors, permitting activation of programmed cell death in neoplastic cells dependent upon suppression of pro-apoptotic pathways. Recently, it was found that bortezomib caused a rapid and dramatic change in the levels of intracellular peptides that are produced by the proteasome. [2] Some intracellular peptides have been shown to be biologically active, and so the effect of bortezomib on the levels of intracellular peptides may contribute to the biological and/or side effects of the drug.
A potent (Ki = 0.6 nM), specific and reversible proteasome inhibitor. It inhibits cell proliferation of H460 cells (Human non-small cell lung cancer cell lines) with an IC₅₀ of 0.1 µM.
References:
1. Takimoto CH, Calvo E. "Principles of Oncologic Pharmacotherapy" in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer Management: A Multidisciplinary Approach. 11 ed. 2008.
2. Gelman JS, Sironi J, Berezniuk I, Dasgupta S, Castro LM, Gozzo FC, Ferro ES, Fricker LD (2013). "Alterations of the intracellular peptidome in response to the proteasome inhibitor bortezomib". In Gartel, Andrei L. PLoS One 8 (8): e53263.
- Sugetriol 6,9-diacetate
Catalog No.:BCN6960
CAS No.:17928-63-1
- Src I1
Catalog No.:BCC7733
CAS No.:179248-59-0
- Zearalenone
Catalog No.:BCC7831
CAS No.:17924-92-4
- Myricitrin
Catalog No.:BCN1136
CAS No.:17912-87-7
- N-Arachidonylglycine
Catalog No.:BCC7069
CAS No.:179113-91-8
- Curzerene
Catalog No.:BCN2352
CAS No.:17910-09-7
- H-Asp(OtBu)-OtBu.HCl
Catalog No.:BCC2893
CAS No.:1791-13-5
- PHCCC
Catalog No.:BCC6895
CAS No.:179068-02-1
- CPCCOEt
Catalog No.:BCC6896
CAS No.:179067-99-3
- FT-207 (NSC 148958)
Catalog No.:BCC4455
CAS No.:17902-23-7
- 3-(4-Pyridyl)-Alanine
Catalog No.:BCC2651
CAS No.:178933-04-5
- NGD 94-1
Catalog No.:BCC7636
CAS No.:178928-68-2
- Eucomol
Catalog No.:BCN6820
CAS No.:17934-12-2
- 7-O-Methyleucomol
Catalog No.:BCN6830
CAS No.:17934-15-5
- Sumanirole maleate
Catalog No.:BCC4112
CAS No.:179386-44-8
- Macrocarpal H
Catalog No.:BCN1137
CAS No.:179388-53-5
- Macrocarpal I
Catalog No.:BCN1138
CAS No.:179388-54-6
- AH 11110 hydrochloride
Catalog No.:BCC6883
CAS No.:179388-65-9
- SDZ 220-581 hydrochloride
Catalog No.:BCC4157
CAS No.:179411-93-9
- SDZ 220-581 Ammonium salt
Catalog No.:BCC1940
CAS No.:179411-94-0
- 2-Methoxyanofinic acid
Catalog No.:BCN7632
CAS No.:179457-70-6
- Leucodin
Catalog No.:BCN7105
CAS No.:17946-87-1
- PD 151746
Catalog No.:BCC5485
CAS No.:179461-52-0
- Caspofungin Acetate
Catalog No.:BCC4895
CAS No.:179463-17-3
Phase II 2-arm trial of the proteasome inhibitor, PS-341 (bortezomib) in combination with irinotecan or PS-341 alone followed by the addition of irinotecan at time of progression in patients with locally recurrent or metastatic squamous cell carcinoma of the head and neck (E1304): a trial of the Eastern Cooperative Oncology Group.[Pubmed:22791234]
Head Neck. 2013 Jul;35(7):942-8.
BACKGROUND: Constitutive activation of nuclear factor kappaB (NF-kappaB) is associated with poor prognosis. Irinotecan demonstrates single-agent activity in head and neck cancer but activates NF-kappaB, promoting cell survival and resistance. Bortezomib is a proteasome inhibitor that inactivates NF-kappaB. PATIENTS AND METHODS: We performed a randomized phase II trial of bortezomib on days 1, 4, 8, and 11 and irinotecan on days 1 and 8 of each 21-day cycle or single-agent bortezomib on days 1, 4, 8, and 11 on a 21-day cycle. The addition of irinotecan to bortezomib was allowed in patients who progressed on bortezomib alone. RESULTS: The response rate of bortezomib and irinotecan was 13%. One patient had a partial response to bortezomib alone (response rate 3%). No responses were seen in patients with addition of irinotecan at time of progression on bortezomib. CONCLUSIONS: The bortezomib-based regimens evaluated in this study have minimal activity in recurrent or metastatic head and neck cancer.
Bortezomib (PS-341) treatment decreases inflammation and partially rescues the expression of the dystrophin-glycoprotein complex in GRMD dogs.[Pubmed:23579193]
PLoS One. 2013 Apr 8;8(4):e61367.
Golden retriever muscular dystrophy (GRMD) is a genetic myopathy corresponding to Duchenne muscular dystrophy (DMD) in humans. Muscle atrophy is known to be associated with degradation of the dystrophin-glycoprotein complex (DGC) via the ubiquitin-proteasome pathway. In the present study, we investigated the effect of bortezomib treatment on the muscle fibers of GRMD dogs. Five GRMD dogs were examined; two were treated (TD- Treated dogs) with the proteasome inhibitor bortezomib, and three were control dogs (CD). Dogs were treated with bortezomib using the same treatment regimen used for multiple myeloma. Pharmacodynamics were evaluated by measuring the inhibition of 20S proteasome activity in whole blood after treatment and comparing it to that in CD. We performed immunohistochemical studies on muscle biopsy specimens to evaluate the rescue of dystrophin and dystrophin-associated proteins in the muscles of GRMD dogs treated with bortezomib. Skeletal tissue from TD had lower levels of connective tissue deposition and inflammatory cell infiltration than CD as determined by histology, collagen morphometry and ultrastructural analysis. The CD showed higher expression of phospho-NFkappaB and TGF-beta1, suggesting a more pronounced activation of anti-apoptotic factors and inflammatory molecules and greater connective tissue deposition, respectively. Immunohistochemical analysis demonstrated that dystrophin was not present in the sarcoplasmic membrane of either group. However, bortezomib-TD showed higher expression of alpha- and beta-dystroglycan, indicating an improved disease histopathology phenotype. Significant inhibition of 20S proteasome activity was observed 1 hour after bortezomib administration in the last cycle when the dose was higher. Proteasome inhibitors may thus improve the appearance of GRMD muscle fibers, lessen connective tissue deposition and reduce the infiltration of inflammatory cells. In addition, proteasome inhibitors may rescue some dystrophin-associated proteins in the muscle fiber membrane.
Two cycles of the PS-341/bortezomib, adriamycin, and dexamethasone combination followed by autologous hematopoietic cell transplantation in newly diagnosed multiple myeloma patients.[Pubmed:22364526]
Eur J Haematol. 2012 Jun;88(6):478-84.
INTRODUCTION: Treatment with 3-6 cycles of PS-341/bortezomib, adriamycin, and dexamethasone (PAD) has been explored in terms of induction therapy prior to autologous stem cell transplantation (ASCT) in patients with multiple myeloma (MM). We evaluated the effects of two cycles of PAD given before ASCT. PATIENTS AND METHODS: Patients received two 21-d cycles of PAD (bortezomib 1.3 mg/m(2) x 4 d, adriamycin 9 mg/m(2) x 4 d, and dexamethasone 40 mg x 4 d x 2). Starting on day 12 of cycle 2, patients were given subcutaneous granulocyte-colony stimulating factor to mobilize peripheral blood stem cells (PBSCs). Following PBSC harvesting, ASCT was performed using high-dose melphalan, followed by thalidomide. RESULTS: A total of 32 patients were enrolled. Of 31 who completed two cycles of PAD, 25 (81%) achieved a partial response (PR) or better. Major adverse events were cytopenia, with grade I/II neurotoxicity evident during 4.8% of PAD cycles. Two patients were withdrawn from the study prior to PBSC collection. Thirty patients showed successful mobilization of PBSCs and underwent ASCT, with all 30 showing adequate neutrophil and platelet recovery. Following ASCT, 14 patients (47%) achieved a complete response (CR), 8 (27%) a very good partial response (VGPR), and 6 (20%) PR. Thalidomide was given to 25 patients after ASCT, as maintenance therapy. Twelve patients showed better responses after administration of thalidomide, and a total of 21 patients (70%) achieved CR. The 5-yr probabilities of overall and progression-free survival were 71.1% and 23.5%, respectively. CONCLUSION: A short course of PAD was effective as an induction treatment before ASCT in patients newly diagnosed with MM. Prospective comparisons with longer courses of such treatment are needed.
Phase I trial of bortezomib (PS-341; NSC 681239) and "nonhybrid" (bolus) infusion schedule of alvocidib (flavopiridol; NSC 649890) in patients with recurrent or refractory indolent B-cell neoplasms.[Pubmed:25248382]
Clin Cancer Res. 2014 Nov 15;20(22):5652-62.
PURPOSE: This phase I study was conducted to determine the dose-limiting toxicities (DLT) and maximum tolerated dose (MTD) for the combination of bortezomib and alvocidib in patients with B-cell malignancies (multiple myeloma, indolent lymphoma, Waldenstrom macroglobulinemia, and mantle cell lymphoma). EXPERIMENTAL DESIGN: Patients received bortezomib (intravenous push), followed by alvocidib (1-hour infusion), on days 1, 4, 8, and 11 of a 21-day treatment cycle. Patients experiencing responses or stable disease continued on treatment at the investigator's discretion. A standard 3+3 dose-escalation design was used to identify the MTD based on DLTs, and pharmacokinetic and pharmacodynamic studies were conducted. RESULTS: A total of 44 patients were enrolled, with 39 patients assessed for response. The MTD was established as 1.3 mg/m(2) for bortezomib and 40 mg/m(2) for alvocidib. The most common hematologic toxicities included leukopenia, lymphopenia, neutropenia, and thrombocytopenia. The most common nonhematologic toxicities included diarrhea, fatigue, and sensory neuropathy. Three complete remissions (8%) and 10 partial remissions (26%) were observed for a total response rate of 33%. Pharmacokinetic findings with the current dosing regimen were consistent with the comparable literature and the hybrid dosing regimen. Pharmacodynamic study results did not correlate with clinical responses. CONCLUSIONS: The combination of bortezomib and alvocidib is tolerable, and an MTD has been established for this schedule. The regimen appears to be efficacious in patients with relapsed/refractory multiple myeloma or indolent non-Hodgkin lymphoma. As the nonhybrid regimen is less cumbersome than the previous hybrid dosing schedule regimen, the current schedule is recommended for successor studies.


