CPCCOEtSelective non-competitive mGlu1 receptor antagonist CAS# 179067-99-3 |
- AM630
Catalog No.:BCC1353
CAS No.:164178-33-0
- Nepicastat
Catalog No.:BCC1795
CAS No.:173997-05-2
- JWH 073
Catalog No.:BCC1674
CAS No.:208987-48-8
- Otenabant
Catalog No.:BCC1828
CAS No.:686344-29-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 179067-99-3 | SDF | Download SDF |
| PubChem ID | 6278000 | Appearance | Powder |
| Formula | C13H13NO4 | M.Wt | 247.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mM in ethanol and to 100 mM in DMSO | ||
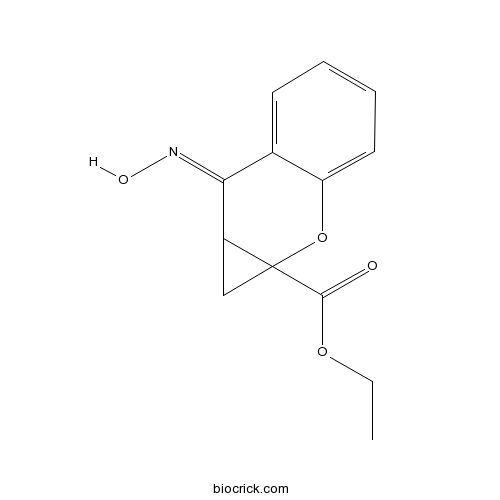
| Chemical Name | ethyl (7E)-7-hydroxyimino-1,7a-dihydrocyclopropa[b]chromene-1a-carboxylate | ||
| SMILES | CCOC(=O)C12CC1C(=NO)C3=CC=CC=C3O2 | ||
| Standard InChIKey | FXCTZFMSAHZQTR-KAMYIIQDSA-N | ||
| Standard InChI | InChI=1S/C13H13NO4/c1-2-17-12(15)13-7-9(13)11(14-16)8-5-3-4-6-10(8)18-13/h3-6,9,16H,2,7H2,1H3/b14-11- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | hmGlu1 subtype-selective non-competitive antagonist (IC50 = 6.5 μM). Has no agonist or antagonist activity at hmGlu2, 4a, 5a, 7b, 8a or ionotropic receptors at concentrations of up to 100 μM. |

CPCCOEt Dilution Calculator

CPCCOEt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0445 mL | 20.2224 mL | 40.4449 mL | 80.8898 mL | 101.1122 mL |
| 5 mM | 0.8089 mL | 4.0445 mL | 8.089 mL | 16.178 mL | 20.2224 mL |
| 10 mM | 0.4044 mL | 2.0222 mL | 4.0445 mL | 8.089 mL | 10.1112 mL |
| 50 mM | 0.0809 mL | 0.4044 mL | 0.8089 mL | 1.6178 mL | 2.0222 mL |
| 100 mM | 0.0404 mL | 0.2022 mL | 0.4044 mL | 0.8089 mL | 1.0111 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- FT-207 (NSC 148958)
Catalog No.:BCC4455
CAS No.:17902-23-7
- 3-(4-Pyridyl)-Alanine
Catalog No.:BCC2651
CAS No.:178933-04-5
- NGD 94-1
Catalog No.:BCC7636
CAS No.:178928-68-2
- Salviaflaside
Catalog No.:BCN8330
CAS No.:178895-25-5
- Myricadiol
Catalog No.:BCN1135
CAS No.:17884-88-7
- Vitexolide D
Catalog No.:BCN6739
CAS No.:1788090-69-6
- SNC 162
Catalog No.:BCC7103
CAS No.:178803-51-5
- 12-Hydroxyisobakuchiol
Catalog No.:BCN3609
CAS No.:178765-55-4
- 3-Hydroxybakuchiol
Catalog No.:BCN3610
CAS No.:178765-54-3
- Delta3,2-Hydroxylbakuchiol
Catalog No.:BCN3707
CAS No.:178765-49-6
- Agrocybenine
Catalog No.:BCN4755
CAS No.:178764-92-6
- rel-(8R,8'R)-dimethyl-(7S,7'R)-bis(3,4-methylenedioxyphenyl)tetrahydro-furan
Catalog No.:BCN1521
CAS No.:178740-32-4
- PHCCC
Catalog No.:BCC6895
CAS No.:179068-02-1
- H-Asp(OtBu)-OtBu.HCl
Catalog No.:BCC2893
CAS No.:1791-13-5
- Curzerene
Catalog No.:BCN2352
CAS No.:17910-09-7
- N-Arachidonylglycine
Catalog No.:BCC7069
CAS No.:179113-91-8
- Myricitrin
Catalog No.:BCN1136
CAS No.:17912-87-7
- Zearalenone
Catalog No.:BCC7831
CAS No.:17924-92-4
- Src I1
Catalog No.:BCC7733
CAS No.:179248-59-0
- Sugetriol 6,9-diacetate
Catalog No.:BCN6960
CAS No.:17928-63-1
- Bortezomib (PS-341)
Catalog No.:BCC1238
CAS No.:179324-69-7
- Eucomol
Catalog No.:BCN6820
CAS No.:17934-12-2
- 7-O-Methyleucomol
Catalog No.:BCN6830
CAS No.:17934-15-5
- Sumanirole maleate
Catalog No.:BCC4112
CAS No.:179386-44-8
Interaction of CPCCOEt with a chimeric mGlu1b and calcium sensing receptor.[Pubmed:10716234]
Neuroreport. 1999 Dec 16;10(18):3923-5.
7-Hydroxyiminocyclopropan[b]chromen-1a-carboxylic acid ethyl ester (CPCCOEt) has previously been shown to be a selective non-competitive antagonist at the metabotropic glutamate (mGlu) receptor subtype 1. In this study we have tested the effect of CPCCOEt on mGlu1b, the calcium sensing receptor (CaR) and a chimeric receptor consisting of the agonist binding amino-terminal domain (ATD) of CaR and the seven transmembrane (7TM) domain of mGlu1b (named Ca/1b). CPCCOEt inhibited responses of (S)-glutamic acid and Ca2+ at mGlu1b and Ca/1b, applied at EC50 values, with IC50 values of 10.2 microM and 13.4 microM, respectively, whereas it was weak as an antagonist of Ca2+ at CaR. These data provides strong evidence that CPCCOEt exerts its antagonistic effect on mGlu1 solely by binding to the 7TM domain.
CPCCOEt, a noncompetitive metabotropic glutamate receptor 1 antagonist, inhibits receptor signaling without affecting glutamate binding.[Pubmed:10051528]
Mol Pharmacol. 1999 Mar;55(3):453-61.
Metabotropic glutamate receptors (mGluRs) are a family of G protein-coupled receptors characterized by a large, extracellular N-terminal domain comprising the glutamate-binding site. In the current study, we examined the pharmacological profile and site of action of the non-amino-acid antagonist 7-hydroxyiminocyclopropan[b]chromen-1a-carboxylic acid ethyl ester (CPCCOEt). CPCCOEt selectively inhibited glutamate-induced increases in intracellular calcium at human mGluR1b (hmGluR1b) with an apparent IC50 of 6.5 microM while having no agonist or antagonist activity at hmGluR2, -4a, -5a, -7b, and -8a up to 100 microM. Schild analysis indicated that CPCCOEt acts in a noncompetitive manner by decreasing the efficacy of glutamate-stimulated phosphoinositide hydrolysis without affecting the EC50 value or Hill coefficient of glutamate. Similarly, CPCCOEt did not displace [3H]glutamate binding to membranes prepared from mGluR1a-expressing cells. To elucidate the site of action, we systematically exchanged segments and single amino acids between hmGluR1b and the related subtype, hmGluR5a. Substitution of Thr815 and Ala818, located at the extracellular surface of transmembrane segment VII, with the homologous amino acids of hmGluR5a eliminated CPCCOEt inhibition of hmGluR1b. In contrast, introduction of Thr815 and Ala818 at the homologous positions of hmGluR5a conferred complete inhibition by CPCCOEt (IC50 = 6.6 microM), i.e., a gain of function. These data suggest that CPCCOEt represents a novel class of G protein-coupled receptor antagonists inhibiting receptor signaling without affecting ligand binding. We propose that the interaction of CPCCOEt with Thr815 and Ala818 of mGluR1 disrupts receptor activation by inhibiting an intramolecular interaction between the agonist-bound extracellular domain and the transmembrane domain.
The mGlu1 antagonist CPCCOEt enhances the climbing fibre response in Purkinje neurones independently of glutamate receptors.[Pubmed:17045308]
Neuropharmacology. 2007 Feb;52(2):450-8.
CPCCOEt (7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester) is frequently used to test for the involvement of mGlu1 receptors. Using whole-cell voltage recording from Purkinje cells in slices of rat cerebellum we find that CPCCOEt, at concentrations used to block mGlu1 receptors, causes an enhancement of the climbing fibre response. Application of alternative antagonists with activity at mGlu1 neither mimicked nor occluded the effects of CPCCOEt. Receptor antagonists demonstrated that this non-mGlu1 action of CPCCOEt was not mediated by other mGlu receptors or GABA(B) receptors. Voltage-clamped climbing fibre EPSCs are unaffected by CPCCOEt whilst application of a glutamate transport blocker did not occlude the CPCCOEt effect. This suggests that a postsynaptic voltage-dependent component of the complex climbing fibre response is the target. We have found no evidence for the involvement of the hyperpolarisation-activated current, I(h), and calcium-activated conductances. Voltage-gated sodium, calcium and potassium channels are possible targets with inhibition of a potassium channel the most likely. Awareness of this non-mGlu-mediated effect of CPCCOEt is likely to be important for the correct interpretation of its actions.
The non-competitive metabotropic glutamate receptor-1 antagonist CPCCOEt inhibits the in vitro growth of human melanoma.[Pubmed:17487397]
Oncol Rep. 2007 Jun;17(6):1399-404.
Five decades ago, the dicarboxylic amino acid glutamate became recognized as the major excitatory neurotransmitter in the central nervous system. In recent years, the expression of glutamate receptors was detected also in peripheral, non-neuronal tissues. Furthermore, it was found that glutamate stimulated the proliferation and migration of several peripheral tumor cells, and that glutamate receptor antagonists limited tumor growth. Most of these studies, however, used broad spectrum compounds and/or group-specific antagonists. Here we report that a selective, non-competitive metabotropic glutamate receptor-1 antagonist, CPCCOEt (7-hydroxyiminocyclopropan[b]chromen-1a-carboxylic acid ethyl ester), significantly inhibited the proliferation and modified the morphology of two human melanoma cell lines. These effects were independent of the external glutamate level in the culture medium. In addition, CPCCOEt significantly enhanced the tumoricidal effects of cytostatic drugs. Thus, selective non-competitive metabotropic glutamate receptor antagonists may be used alone and/or with the synergistic effects of chemotherapy, thus enhancing existing therapies of melanoma and possibly other malignancies.
Reversible and non-competitive antagonist profile of CPCCOEt at the human type 1alpha metabotropic glutamate receptor.[Pubmed:9886688]
Neuropharmacology. 1998 Dec;37(12):1645-7.
In transfected CHO cells expressing the human metabotropic glutamate receptor mGlu1alpha, 7-(hydroxyimino)cyclopropan[b]-chromen-1a-carboxylic acid ethylester (CPCCOEt) was found to antagonize L-quisqualate-induced phosphoinositide hydrolysis in a non-competitive and reversible manner (apparent pKi value, 4.76+/-0.18; n=3). This suggests that CPCCOEt antagonizes type 1alpha metabotropic glutamate receptor activation by interacting with a site distinct from the agonist binding site.


