SNC 162CAS# 178803-51-5 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 178803-51-5 | SDF | Download SDF |
| PubChem ID | 6604878 | Appearance | Powder |
| Formula | C27H37N3O | M.Wt | 419.61 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. HCl | ||
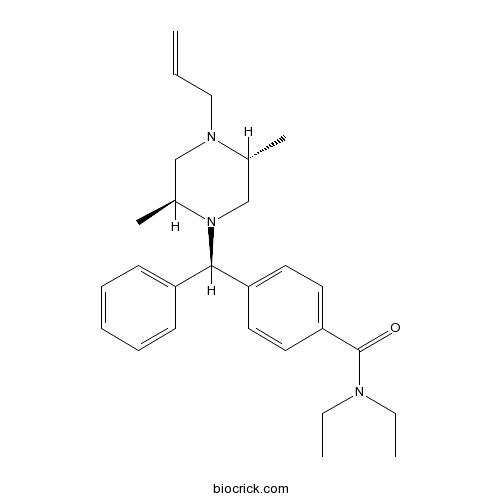
| Chemical Name | 4-[(S)-[(2S,5R)-2,5-dimethyl-4-prop-2-enylpiperazin-1-yl]-phenylmethyl]-N,N-diethylbenzamide | ||
| SMILES | CCN(CC)C(=O)C1=CC=C(C=C1)C(C2=CC=CC=C2)N3CC(N(CC3C)CC=C)C | ||
| Standard InChIKey | WGIDFDFAOQVAHY-UFPGJGBJSA-N | ||
| Standard InChI | InChI=1S/C27H37N3O/c1-6-18-29-19-22(5)30(20-21(29)4)26(23-12-10-9-11-13-23)24-14-16-25(17-15-24)27(31)28(7-2)8-3/h6,9-17,21-22,26H,1,7-8,18-20H2,2-5H3/t21-,22+,26+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective non-peptide δ-opioid receptor agonist (Ki = 0.63 nM). Displays > 8000-fold selectivity over μ-opioid receptors and is centrally active following systemic administration in vivo. |

SNC 162 Dilution Calculator

SNC 162 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3832 mL | 11.9158 mL | 23.8317 mL | 47.6633 mL | 59.5791 mL |
| 5 mM | 0.4766 mL | 2.3832 mL | 4.7663 mL | 9.5327 mL | 11.9158 mL |
| 10 mM | 0.2383 mL | 1.1916 mL | 2.3832 mL | 4.7663 mL | 5.9579 mL |
| 50 mM | 0.0477 mL | 0.2383 mL | 0.4766 mL | 0.9533 mL | 1.1916 mL |
| 100 mM | 0.0238 mL | 0.1192 mL | 0.2383 mL | 0.4766 mL | 0.5958 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 12-Hydroxyisobakuchiol
Catalog No.:BCN3609
CAS No.:178765-55-4
- 3-Hydroxybakuchiol
Catalog No.:BCN3610
CAS No.:178765-54-3
- Delta3,2-Hydroxylbakuchiol
Catalog No.:BCN3707
CAS No.:178765-49-6
- Agrocybenine
Catalog No.:BCN4755
CAS No.:178764-92-6
- rel-(8R,8'R)-dimethyl-(7S,7'R)-bis(3,4-methylenedioxyphenyl)tetrahydro-furan
Catalog No.:BCN1521
CAS No.:178740-32-4
- CFM-2
Catalog No.:BCC6931
CAS No.:178616-26-7
- U-104
Catalog No.:BCC2312
CAS No.:178606-66-1
- Oleoside
Catalog No.:BCN1134
CAS No.:178600-68-5
- ZD 2079
Catalog No.:BCC5878
CAS No.:178600-17-4
- Prilocaine hydrochloride
Catalog No.:BCC4288
CAS No.:1786-81-8
- Hoechst 33342 analog
Catalog No.:BCC1630
CAS No.:178481-68-0
- Vitexin -4''-O-glucoside
Catalog No.:BCN3054
CAS No.:178468-00-3
- Vitexolide D
Catalog No.:BCN6739
CAS No.:1788090-69-6
- Myricadiol
Catalog No.:BCN1135
CAS No.:17884-88-7
- Salviaflaside
Catalog No.:BCN8330
CAS No.:178895-25-5
- NGD 94-1
Catalog No.:BCC7636
CAS No.:178928-68-2
- 3-(4-Pyridyl)-Alanine
Catalog No.:BCC2651
CAS No.:178933-04-5
- FT-207 (NSC 148958)
Catalog No.:BCC4455
CAS No.:17902-23-7
- CPCCOEt
Catalog No.:BCC6896
CAS No.:179067-99-3
- PHCCC
Catalog No.:BCC6895
CAS No.:179068-02-1
- H-Asp(OtBu)-OtBu.HCl
Catalog No.:BCC2893
CAS No.:1791-13-5
- Curzerene
Catalog No.:BCN2352
CAS No.:17910-09-7
- N-Arachidonylglycine
Catalog No.:BCC7069
CAS No.:179113-91-8
- Myricitrin
Catalog No.:BCN1136
CAS No.:17912-87-7
Opioid receptors and the discriminative stimulus effects of ethanol in squirrel monkeys: Mu and delta opioid receptor mechanisms.[Pubmed:20940013]
Eur J Pharmacol. 2011 Jan 10;650(1):233-9.
Mu and delta opioid receptors modulate the reinforcing effects of ethanol, however, their role in the subjective effects of ethanol is not well understood. This study evaluated the contribution of mu and delta opioid receptors to the subjective effects of ethanol using drug discrimination procedures. Monkeys were trained to discriminate ethanol from saline under a schedule of food delivery. In tests, ethanol engendered increases in drug-lever responding, reaching a maximum of >80%. The mu opioid receptor agonists fentanyl and buprenorphine and the delta opioid receptor agonists SNC 80 and SNC 162 did not substitute for the discriminative stimulus effects of ethanol. As pretreatments, the full agonists fentanyl and SNC 80 enhanced the effects of low doses of ethanol and fentanyl attenuated the effects of the ethanol training dose. Although the possibility of pharmacological antagonism of the effects of ethanol cannot be ruled out, a more likely alternative is that the diminished effects of ethanol were due to perceptual masking of the ethanol stimulus. In contrast, the partial agonists buprenorphine and SNC 162 did not alter ethanol's effects. Finally, the discriminative stimulus effects of ethanol were attenuated following administration of presumably mu-selective doses of the antagonist naltrexone, but not after administration of the delta opioid receptor antagonist naltrindole. The ability of naltrexone to block the discriminative stimulus effects of ethanol likely reflects its capacity to attenuate ethanol-induced increases in endogenous opioids, in particular beta-endorphin, because attenuation of the ethanol stimulus was not accompanied by significant suppression of response rate.
Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys.[Pubmed:9655881]
J Pharmacol Exp Ther. 1998 Jul;286(1):362-75.
The behavioral effects of the nonpeptidic delta opioid agonist SNC80 and a series of related piperazinyl benzamides derived from the parent compound BW373U86 were evaluated in rhesus monkeys. SNC80 (0.1-10 mg/kg) decreased response rates maintained by food-reinforcement in a dose- and time-dependent manner, with maximal effects occurring within 10 min of intramuscular injection. The potency of SNC80 and five other piperazinyl benzamides in this assay of schedule-controlled responding correlated with their affinity at cloned human delta opioid receptors but not with their affinity for cloned human mu receptors. Moreover, the effects of SNC80 were selectively antagonized by the delta-selective antagonist naltrindole (1.0 mg/kg), but not by the mu selective antagonist quadazocine (0.1 mg/kg) or the kappa-selective antagonist norbinaltorphimine (3.2 mg/kg). These findings indicate that SNC80 functions as a systemically active, delta-selective agonist with a rapid onset of action in rhesus monkeys. The antinociceptive effects of SNC80 were examined in a warm-water tail-withdrawal assay of thermal nociception. SNC80 (0.1-10 mg/kg) produced weak but replicable antinociceptive effects that were antagonized by naltrindole (1.0 mg/kg). SNC80 antinociception was also dose-dependently antagonized by BW373U86 (0.56-1.0 mg/kg), which was inactive in this procedure. These findings suggest that SNC80 may have higher efficacy than BW373U86 at delta opioid receptors. Moreover, SNC80 at doses up to 32 mg/kg did not produce convulsions, which suggests that SNC80 may also be safer than BW373U86. The effects of SNC80 were also examined in monkeys trained to discriminate cocaine (0.4 mg/kg i.m.) or self-administer cocaine (0.032 mg/kg/injection,i.v.). In drug discrimination studies, SNC80 (0.1-10 mg/kg) produced a dose-dependent and naltrindole-reversible increase in cocaine-appropriate responding, and complete substitution for cocaine was observed in five of seven monkeys tested. However, SNC80 (1.0-100 micrograms/kg/injection) did not maintain responding in monkeys trained to self-administer cocaine. Thus, despite its ability to produce cocaine-like discriminative stimulus effects, SNC80 may have relatively low abuse potential.
Probes for narcotic receptor mediated phenomena. 23. Synthesis, opioid receptor binding, and bioassay of the highly selective delta agonist (+)-4-[(alpha R)-alpha-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]- N,N-diethylbenzamide (SNC 80) and related novel nonpeptide delta opioid receptor ligands.[Pubmed:9057856]
J Med Chem. 1997 Feb 28;40(5):695-704.
The highly selective delta (delta) opioid receptor agonist SNC 80 [(+)-4- [(alpha R)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N ,N- diethylbenzamide, (+)-21] and novel optically pure derivatives were synthesized from the enantiomers of 1-allyl-trans-2,5-dimethylpiperazine (2). The piperazine (+/-)-2 was synthesized, and its enantiomers were obtained on a multigram scale in > 99% optical purity by optical resolution of the racemate with the camphoric acids. The absolute configuration of (+)-2 was determined to be 2S,5R by X-ray analysis of the salt with (+)-camphoric acid. Since the chirality of the starting material was known, and the relative configuration of compounds (-)-21, (-)-22, and (+)-23 were obtained by single-crystal X-ray analysis, the assignment of the absolute stereochemistry of the entire series could be made. Radioreceptor binding studies in rat brain preparations showed that methyl ethers (+)-21 (SNC 80) and (-)-25 exhibited strong selectivity for rat delta receptors with low nanomolar affinity to delta receptors and only micromolar affinity for rat mu (mu) opioid receptors. Compounds (-)-21, (-)-22, and (-)-23 showed micromolar affinities for delta opioid receptors. The unsubstituted derivative (+)-22 and the fluorinated derivative (-)-27 showed > 2659- and > 2105-fold delta/mu binding selectivity, respectively. The latter derivatives are the most selective ligands described in the new series. Studies with some of the compounds described in the isolated mouse vas deferens and guinea pig ileum bioassays revealed that all were agonists with different degrees of selectivity for the delta opioid receptor. These data show that (+)-21 and (+)-22 are potent delta receptor agonists and suggest that these compounds will be valuable tools for further study of the delta opioid receptor at the molecular level, including its function and role in analgesia and drug abuse.
Structure-activity relationships for SNC80 and related compounds at cloned human delta and mu opioid receptors.[Pubmed:8667189]
J Pharmacol Exp Ther. 1996 Jun;277(3):1284-91.
The racemic compound (+/-)-BW373U86 inverted question mark(+/-)-4-((alpha R*)- alpha-((2S*,5R*)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-hydroxy- benzyl)-N,N-diethylbenzamide dihydrochloride} is a potent delta opioid receptor agonist in the mouse vas deferens assay with little mu or kappa opioid receptor activity in the guinea pig ileum tissue preparation. In contrast, radioligand binding studies show that (+/-)-BW373U86 is only about 10-fold selective for delta over mu opioid receptors. Studies of the enantiomeric forms of (+/-)-BW373U86 and derivatives (SNC80 and related compounds) show that some of these isomers are significantly better in both receptor binding and pharmacological selectivity than (+/-)-BW373U86. In this study we have determined the binding affinities of 10 different SNC80-related compounds at cloned human delta and mu opioid receptors and measured the potency of SNC80 for the inhibition of forskolin-stimulated adenylyl cyclase. The most selective delta receptor ligand (SNC162) differed from SNC80 by the absence of the 3-methoxy substitution of the benzyl ring. The Ki for SNC162 at the delta receptor (0.625 nM) was over 8700-fold lower than that at the mu receptor (5500 nM), making this the most selective delta receptor ligand available. Reduction of the allyl side chain of SNC80 to produce radiolabeled [3H]SNC121 allowed direct measurement of the association and dissociation rate constants. SNC80 was 26-fold less potent than [D-Pen2, pCI-Phe4, D-Pen5]enkephalin in the delta receptor adenylyl cyclase inhibition assay, but showed full agonist activity with an EC50 value of 9.2 nM. The regulation of SNC80 binding affinity to the delta receptor by GTP analogs is undetectable in [3H]naltrindole binding inhibition studies, but direct binding studies with [3H]SNC121 in the presence of 100 microM 5'-guanylylimidotriphosphate show a 55% reduction in maximum binding site density consistent with a lower affinity for a part of the receptor population. Addition of 120 mM sodium chloride reduces SNC80 affinity nearly 40-fold in [3H]naltrindole binding inhibition studies. The results of these studies define specific structural features of these compounds responsible for opioid receptor interactions and suggest a possibly novel mechanism for delta receptor activation.


