Hoechst 33342 analogBlue fluorescent dyes CAS# 178481-68-0 |
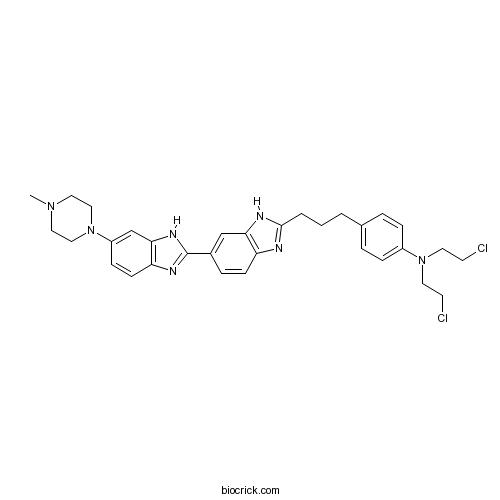
- Hoechst 33342 analog 2
Catalog No.:BCC1631
CAS No.:106050-84-4
- Hoechst 33342
Catalog No.:BCC1629
CAS No.:23491-52-3
- Hoechst 33258 analog 2
Catalog No.:BCC1625
CAS No.:23491-54-5
- Hoechst 33258 analog 5
Catalog No.:BCC1627
CAS No.:23491-55-6
- Hoechst 34580
Catalog No.:BCC1632
CAS No.:23555-00-2
- Hoechst 33258 analog
Catalog No.:BCC1624
CAS No.:258843-62-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 178481-68-0 | SDF | Download SDF |
| PubChem ID | 197366 | Appearance | Powder |
| Formula | C32H37Cl2N7 | M.Wt | 590.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | 25℃: DMSO or water Protect from light | ||
| Chemical Name | N,N-bis(2-chloroethyl)-4-[3-[6-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-1H-benzimidazol-2-yl]propyl]aniline | ||
| SMILES | CN1CCN(CC1)C2=CC3=C(C=C2)N=C(N3)C4=CC5=C(C=C4)N=C(N5)CCCC6=CC=C(C=C6)N(CCCl)CCCl | ||
| Standard InChIKey | PZEAMYHHAKUANN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C32H37Cl2N7/c1-39-17-19-41(20-18-39)26-10-12-28-30(22-26)38-32(37-28)24-7-11-27-29(21-24)36-31(35-27)4-2-3-23-5-8-25(9-6-23)40(15-13-33)16-14-34/h5-12,21-22H,2-4,13-20H2,1H3,(H,35,36)(H,37,38) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Hoechst 33342 analog is an anglog of Hoechst 33342, which is a DNA minor groove binder used fluorochrome for visualizing cellular DNA. References: | |||||

Hoechst 33342 analog Dilution Calculator

Hoechst 33342 analog Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6932 mL | 8.4661 mL | 16.9322 mL | 33.8644 mL | 42.3306 mL |
| 5 mM | 0.3386 mL | 1.6932 mL | 3.3864 mL | 6.7729 mL | 8.4661 mL |
| 10 mM | 0.1693 mL | 0.8466 mL | 1.6932 mL | 3.3864 mL | 4.2331 mL |
| 50 mM | 0.0339 mL | 0.1693 mL | 0.3386 mL | 0.6773 mL | 0.8466 mL |
| 100 mM | 0.0169 mL | 0.0847 mL | 0.1693 mL | 0.3386 mL | 0.4233 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: N/A Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA. These Bis-benzimides were originally developed by Hoechst AG, which numbered all their compounds so that the dye Hoechst 33342 is the 33342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they have similarexcitation/emission spectra. Both dyes are excited by ultraviolet light at around 350 nm, and both emit blue/cyan fluorescent light around anemission maximum at 461 nm. Unbound dye has its maximum fluorescence emission in the 510-540 nm range. Hoechst dyes are soluble in water and in organic solvents such as dimethyl formamide or dimethyl sulfoxide. Concentrations can be achieved of up to 10 mg/mL. Aqueous solutions are stable at 2-6 °C for at least six months when protected from light. For long-term storage the solutions are instead frozen at ≤-20 °C. The dyes bind to the minor groove of double-stranded DNA with a preference for sequences rich in adenine andthymine. Although the dyes can bind to all nucleic acids, AT-rich double-stranded DNA strands enhance fluorescence considerably. Hoechst dyes are cell-permeable and can bind to DNA in live or fixed cells. Therefore, these stains are often called supravital, which means that cells survive a treatment with these compounds. Cells that express specific ATP-binding cassette transporter proteins can also actively transport these stains out of their cytoplasm. in vitro: N/A in vivo: N/A Clinical trial: N/A
- Vitexin -4''-O-glucoside
Catalog No.:BCN3054
CAS No.:178468-00-3
- 6-epi-Albrassitriol
Catalog No.:BCN7342
CAS No.:178456-58-1
- Agrostophyllidin
Catalog No.:BCN3598
CAS No.:178439-50-4
- AR-R 17779 hydrochloride
Catalog No.:BCC7827
CAS No.:178419-42-6
- Tos-Arg-OMe.HCl
Catalog No.:BCC2874
CAS No.:1784-03-8
- 12-Hydroxy-6-epi-albrassitriol
Catalog No.:BCN7460
CAS No.:178330-78-4
- H-D-Asp-OH
Catalog No.:BCC2894
CAS No.:1783-96-6
- Nociceptin (1-7)
Catalog No.:BCC5738
CAS No.:178249-42-8
- Orphanin FQ (1-11)
Catalog No.:BCC6085
CAS No.:178249-41-7
- Calystegine B3
Catalog No.:BCN1880
CAS No.:178231-95-3
- Tetrahymanone
Catalog No.:BCN6932
CAS No.:17822-06-9
- Linderone
Catalog No.:BCN1133
CAS No.:1782-79-2
- Prilocaine hydrochloride
Catalog No.:BCC4288
CAS No.:1786-81-8
- ZD 2079
Catalog No.:BCC5878
CAS No.:178600-17-4
- Oleoside
Catalog No.:BCN1134
CAS No.:178600-68-5
- U-104
Catalog No.:BCC2312
CAS No.:178606-66-1
- CFM-2
Catalog No.:BCC6931
CAS No.:178616-26-7
- rel-(8R,8'R)-dimethyl-(7S,7'R)-bis(3,4-methylenedioxyphenyl)tetrahydro-furan
Catalog No.:BCN1521
CAS No.:178740-32-4
- Agrocybenine
Catalog No.:BCN4755
CAS No.:178764-92-6
- Delta3,2-Hydroxylbakuchiol
Catalog No.:BCN3707
CAS No.:178765-49-6
- 3-Hydroxybakuchiol
Catalog No.:BCN3610
CAS No.:178765-54-3
- 12-Hydroxyisobakuchiol
Catalog No.:BCN3609
CAS No.:178765-55-4
- SNC 162
Catalog No.:BCC7103
CAS No.:178803-51-5
- Vitexolide D
Catalog No.:BCN6739
CAS No.:1788090-69-6
Combination therapy with telmisartan and oxacalcitriol suppresses the progression of murine adriamycin nephropathy.[Pubmed:25661164]
Nephron. 2015;129(2):143-54.
BACKGROUND: Blockade of the renin-angiotensin system plays a key role in suppressing the progression of renal diseases. It has not been well established whether this therapy provides additional effects when combined with vitamin D or its analog in a model of adriamycin (ADR)-induced nephropathy. METHODS: We evaluated the effect of an angiotensin II subtype 1 receptor blocker (telmisartan) combined with a vitamin D analog (oxacalcitriol) on mice ADR-induced nephropathy (9.5 mg/kg single intravenous injection). We also tested immortalized murine podocytes to examine the effects on podocyte apoptosis. RESULTS: Mice with ADR-induced nephropathy developed progressive albuminuria and glomerulosclerosis within 30 days accompanied by decreased expression of slit diaphragm (SD)-associated proteins (nephrin and podocin), reduced numbers of podocytes, and increased systolic blood pressure. Treatment with telmisartan or oxacalcitriol alone moderately ameliorated kidney injury. The combined treatment most effectively reduced the albuminuria and glomerulosclerosis. These effects were accompanied by the restoration of SD-associated proteins, reduction of podocyte apoptosis, and prevention of podocyte depletion in the glomeruli. Treatment with telmisartan, oxacalcitriol, and the combination therapy resulted in similar reductions in systolic blood pressure. In cultured murine podocytes, ADR stimulated the expression of Bax/Bcl-2 and apoptosis as determined by Hoechst 33342 staining. These changes were effectively inhibited by telmisartan or oxacalcitriol, but the combination treatment most effectively reduced these effects. CONCLUSIONS: These data demonstrated that application of a renin-angiotensin system blocker plus a vitamin D analog effectively prevented renal injury in ADR-induced nephropathy. The observed amelioration of renal injury may be partly attributable to antiapoptotic effects in podocytes.
Application of JC1 for non-toxic isolation of cells with MDR transporter activity by flow cytometry.[Pubmed:28380010]
PLoS One. 2017 Apr 5;12(4):e0174905.
The DNA intercalating dye Hoechst 33342 or its close analog DCV are actively removed from cells by the multidrug resistance transporter ABCG2, a protein overexpressed in metastatic cells and somatic stem cells. In bivariate blue-red flow cytometry fluorescent plots active Hoechst or DCV efflux combined with a concentration dependent bathochromic shifts of these nuclear dyes leads to the segregation of the transporter-rich cells into a distinct cell cohort tilted towards the shorter wavelength axis of the plot, the cohort is generically known as the side population (SP). This feature has facilitated the surface marker-independent isolation of live stem cells. A drawback, though, is the known toxicity of Hoechst dyes. In this study we show that JC1, a bathochromic mitochondrial membrane potential-sensitive dye applied at proper concentration, can yield flow cytometry fluorescent emission bivariate plots containing a low JC1 accumulation (JC1low) cohort. Using a combination of multiple cell lines, ABC-transporter inhibitors and viral vector-driven insertion of the ABCG2 gene or ABCG2 and ABCB1 shRNAs we demonstrate that JC1low can be generated by either of the two aforementioned multidrug resistance transporters. Complete wash out of mitochondrial bound JC1 required more than 24 h. In spite of this tight binding, the dye did not affect either the mitochondrial membrane potentials or the proliferation rate. In contrast, contemporaneous with its nuclear accumulation, Hoechst 33342 or DVC, caused changes in the fluorescent emission of mitochondrial membrane potential sensitive dyes resembling the effects caused by the mitochondrial uncoupler FCCP. In a number of cell lines exposure to Hoechst resulted in marked slow-down of proliferation and abolition of ABCG2 transport activity during the subsequent 2 days but in K562 cells the exposure induced cell extended death. Overall, its lack of toxicity vis. a vis. the toxicity and genotoxicity of the DNA intercalating dyes makes JC1 an ideal tool for isolating live cells expressing high multidrug resistance transport activity.
The apoptotic pathways in the curcumin analog MHMD-induced lung cancer cell death and the essential role of actin polymerization during apoptosis.[Pubmed:25960227]
Biomed Pharmacother. 2015 Apr;71:128-34.
As a mode of cell death, apoptosis could be triggered by the extrinsic, intrinsic mitochondrial and intrinsic endoplasmic reticulum pathways and actin rearrangement is needed during apoptosis. We previously found that one curcumin analog MHMD could induce A549 lung cancer cells apoptosis. But the apoptotic pathways and the actin dynamics during apoptosis are not known. Here, we detected the activation of caspase-3, -8, -9, -12, PARP and the increase ratio of Bax/Bcl-2 by western blotting in MHMD-exposed A549 cells. Alternatively, caspases inhibitors could lead to the disappearance of MHMD-eliciting nuclei fragmentation by Hoechst 33342 staining. Besides, JC-1 and DCFH-DA staining showed the fall of mitochondrial membrane potential and the release of ROS. Moreover, wound healing assay confirmed the MHMD anti-migration ability, which was much more effective than curcumin. Importantly, unlike other anticarcinogenic drugs, MHMD might induce the actin polymerization but not depolymerization in the process of A549 cell apoptosis by phalloidin-FITC staining, which is essential to MHMD-induced extrinsic, intrinsic mitochondrial and intrinsic ER pathways of cell apoptosis.
Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models.[Pubmed:25060424]
Cancer Sci. 2014 Oct;105(10):1334-42.
Eribulin mesylate is a synthetic macrocyclic ketone analog of the marine sponge natural product halichondrin B and an inhibitor of microtubule dynamics. Some tubulin-binding drugs are known to have antivascular (antiangiogenesis or vascular-disrupting) activities that can target abnormal tumor vessels. Using dynamic contrast-enhanced MRI analyses, here we show that eribulin induces remodeling of tumor vasculature through a novel antivascular activity in MX-1 and MDA-MB-231 human breast cancer xenograft models. Vascular remodeling associated with improved perfusion was shown by Hoechst 33342 staining and by increased microvessel density together with decreased mean vascular areas and fewer branched vessels in tumor tissues, as determined by immunohistochemical staining for endothelial marker CD31. Quantitative RT-PCR analysis of normal host cells in the stroma of xenograft tumors showed that eribulin altered the expression of mouse (host) genes in angiogenesis signaling pathways controlling endothelial cell-pericyte interactions, and in the epithelial-mesenchymal transition pathway in the context of the tumor microenvironment. Eribulin also decreased hypoxia-associated protein expression of mouse (host) vascular endothelial growth factor by ELISA and human CA9 by immunohistochemical analysis. Prior treatment with eribulin enhanced the anti-tumor activity of capecitabine in the MDA-MB-231 xenograft model. These findings suggest that eribulin-induced remodeling of abnormal tumor vasculature leads to a more functional microenvironment that may reduce the aggressiveness of tumors due to elimination of inner tumor hypoxia. Because abnormal tumor microenvironments enhance both drug resistance and metastasis, the apparent ability of eribulin to reverse these aggressive characteristics may contribute to its clinical benefits.
The anti-tumor effects of cordycepin-loaded liposomes on the growth of hepatoma 22 tumors in mice and human hepatoma BEL-7402 cells in culture.[Pubmed:26984179]
Drug Dev Ind Pharm. 2016 Sep;42(9):1424-33.
Liposomes have successfully been used for decades to encapsulate and protect drugs that are prone to deactivation in the body. The present study aimed to demonstrate the use of liposomes to encapsulate cordycepin, an adenosine analog that quickly loses its activity in vivo. The cordycepin-loaded liposomes were prepared by the ammonium sulfate gradient approach, and its in vitro and in vivo antitumour activities were evaluated using BEL-7402 cells and hepatocellular carcinoma H22 transplanted tumors, respectively. An MTT assay was used to observe the cytotoxicity of cells treated with cordycepin and cordycepin-loaded liposomes in vitro. High-content screening (HSC) was carried out using Hoechst 33342 to detect apoptotic cells and the ratio of cells in different cell cycle stages. The data demonstrated that both the cordycepin and the cordycepin-loaded liposomes resulted in clear cytotoxicity with IC50 values of 18.97 and 29.39 mug/mL, respectively. The latter showed significantly strong inhibitory effects on H22 tumor growth in mice, while the former did not show any inhibitory effects on tumor growth. In addition, the HSC assay showed that the cordycepin-loaded liposomes resulted in a higher rate of apoptosis than the cordycepin alone in BEL-7402 cells. Further data analysis revealed that the cells treated with cordycepin-loaded liposomes were predominately arrested at the G2/M phase (p < 0.05), while those treated with cordycepin alone were arrested in the G0/G1 phase (p < 0.05). In conclusion, these results suggest that liposomes can enhance and maintain the in vivo anti-tumor activity of cordycepin.


