Hoechst 33342 analog 2Blue fluorescent dyes CAS# 106050-84-4 |
- Hoechst 33342 analog 2
Catalog No.:BCC1631
CAS No.:106050-84-4
- Hoechst 33342
Catalog No.:BCC1629
CAS No.:23491-52-3
- Hoechst 33258 analog 2
Catalog No.:BCC1625
CAS No.:23491-54-5
- Hoechst 33258 analog 5
Catalog No.:BCC1627
CAS No.:23491-55-6
- Hoechst 34580
Catalog No.:BCC1632
CAS No.:23555-00-2
- Hoechst 33258 analog
Catalog No.:BCC1624
CAS No.:258843-62-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 106050-84-4 | SDF | Download SDF |
| PubChem ID | 5487193 | Appearance | Powder |
| Formula | C25H23IN6O | M.Wt | 550.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 57 mg/mL (103.56 mM); | ||
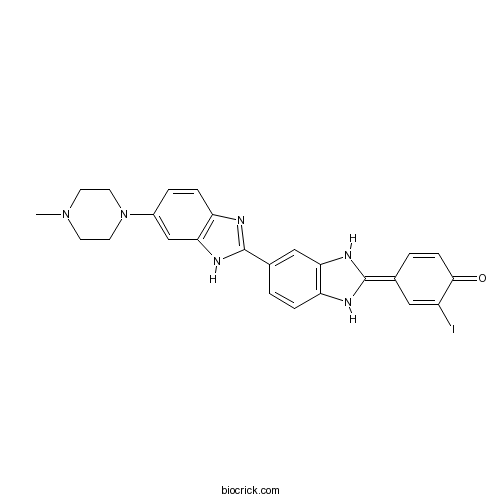
| Chemical Name | (4E)-2-iodo-4-[5-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-1,3-dihydrobenzimidazol-2-ylidene]cyclohexa-2,5-dien-1-one | ||
| SMILES | CN1CCN(CC1)C2=CC3=C(C=C2)N=C(N3)C4=CC5=C(C=C4)NC(=C6C=CC(=O)C(=C6)I)N5 | ||
| Standard InChIKey | ZIZURNQOGKSUHV-BUVRLJJBSA-N | ||
| Standard InChI | InChI=1S/C25H23IN6O/c1-31-8-10-32(11-9-31)17-4-6-20-22(14-17)30-25(28-20)16-2-5-19-21(13-16)29-24(27-19)15-3-7-23(33)18(26)12-15/h2-7,12-14,27,29H,8-11H2,1H3,(H,28,30)/b24-15+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Hoechst 33342 analog 2 is an anglog of Hoechst 33342, which is a DNA minor groove binder used fluorochrome for visualizing cellular DNA. References: | |||||

Hoechst 33342 analog 2 Dilution Calculator

Hoechst 33342 analog 2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8169 mL | 9.0845 mL | 18.1689 mL | 36.3379 mL | 45.4223 mL |
| 5 mM | 0.3634 mL | 1.8169 mL | 3.6338 mL | 7.2676 mL | 9.0845 mL |
| 10 mM | 0.1817 mL | 0.9084 mL | 1.8169 mL | 3.6338 mL | 4.5422 mL |
| 50 mM | 0.0363 mL | 0.1817 mL | 0.3634 mL | 0.7268 mL | 0.9084 mL |
| 100 mM | 0.0182 mL | 0.0908 mL | 0.1817 mL | 0.3634 mL | 0.4542 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: N/A Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA. These Bis-benzimides were originally developed by Hoechst AG, which numbered all their compounds so that the dye Hoechst 33342 is the 33342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they have similarexcitation/emission spectra. Both dyes are excited by ultraviolet light at around 350 nm, and both emit blue/cyan fluorescent light around anemission maximum at 461 nm. Unbound dye has its maximum fluorescence emission in the 510-540 nm range. Hoechst dyes are soluble in water and in organic solvents such as dimethyl formamide or dimethyl sulfoxide. Concentrations can be achieved of up to 10 mg/mL. Aqueous solutions are stable at 2-6 °C for at least six months when protected from light. For long-term storage the solutions are instead frozen at ≤-20 °C. The dyes bind to the minor groove of double-stranded DNA with a preference for sequences rich in adenine andthymine. Although the dyes can bind to all nucleic acids, AT-rich double-stranded DNA strands enhance fluorescence considerably. Hoechst dyes are cell-permeable and can bind to DNA in live or fixed cells. Therefore, these stains are often called supravital, which means that cells survive a treatment with these compounds. Cells that express specific ATP-binding cassette transporter proteins can also actively transport these stains out of their cytoplasm. in vitro: N/A in vivo: N/A Clinical trial: N/A
- Palmatine hydrochloride
Catalog No.:BCN5914
CAS No.:10605-02-4
- β-Interleukin I (163-171), human
Catalog No.:BCC1017
CAS No.:106021-96-9
- Nerol
Catalog No.:BCN8517
CAS No.:106-25-2
- Geraniol
Catalog No.:BCN2631
CAS No.:106-24-1
- 2-(3,4-Dihydroxyphenyl)ethanol
Catalog No.:BCN5871
CAS No.:10597-60-1
- Sulfocostunolide B
Catalog No.:BCN5870
CAS No.:1059671-65-6
- Clinafloxacin CI96 AM1091
Catalog No.:BCC3754
CAS No.:105956-97-6
- OLDA
Catalog No.:BCC7138
CAS No.:105955-11-1
- STEARDA
Catalog No.:BCC7288
CAS No.:105955-10-0
- Doxycycline HCl
Catalog No.:BCC3772
CAS No.:10592-13-9
- Taraxasterol
Catalog No.:BCN5869
CAS No.:1059-14-9
- (tert-Butoxycarbonyl)oxycefcapene pivoxil
Catalog No.:BCC8403
CAS No.:105889-80-3
- Senktide
Catalog No.:BCC6921
CAS No.:106128-89-6
- PND-1186
Catalog No.:BCC1866
CAS No.:1061353-68-1
- TC-G 1004
Catalog No.:BCC6165
CAS No.:1061747-72-5
- WAY-600
Catalog No.:BCC4607
CAS No.:1062159-35-6
- WYE-687
Catalog No.:BCC4604
CAS No.:1062161-90-3
- WYE-354
Catalog No.:BCC1059
CAS No.:1062169-56-5
- Ro3280
Catalog No.:BCC3962
CAS No.:1062243-51-9
- LDN-193189
Catalog No.:BCC3687
CAS No.:1062368-24-4
- ML347
Catalog No.:BCC5331
CAS No.:1062368-49-3
- LDN193189 Hydrochloride
Catalog No.:BCC1695
CAS No.:1062368-62-0
- Thioperamide
Catalog No.:BCC6734
CAS No.:106243-16-7
- 4-[(4-Methylpiperazin-1-yl) methyl]benzoic acid dihydrochloride
Catalog No.:BCC8669
CAS No.:106261-49-8
Combination therapy with telmisartan and oxacalcitriol suppresses the progression of murine adriamycin nephropathy.[Pubmed:25661164]
Nephron. 2015;129(2):143-54.
BACKGROUND: Blockade of the renin-angiotensin system plays a key role in suppressing the progression of renal diseases. It has not been well established whether this therapy provides additional effects when combined with vitamin D or its analog in a model of adriamycin (ADR)-induced nephropathy. METHODS: We evaluated the effect of an angiotensin II subtype 1 receptor blocker (telmisartan) combined with a vitamin D analog (oxacalcitriol) on mice ADR-induced nephropathy (9.5 mg/kg single intravenous injection). We also tested immortalized murine podocytes to examine the effects on podocyte apoptosis. RESULTS: Mice with ADR-induced nephropathy developed progressive albuminuria and glomerulosclerosis within 30 days accompanied by decreased expression of slit diaphragm (SD)-associated proteins (nephrin and podocin), reduced numbers of podocytes, and increased systolic blood pressure. Treatment with telmisartan or oxacalcitriol alone moderately ameliorated kidney injury. The combined treatment most effectively reduced the albuminuria and glomerulosclerosis. These effects were accompanied by the restoration of SD-associated proteins, reduction of podocyte apoptosis, and prevention of podocyte depletion in the glomeruli. Treatment with telmisartan, oxacalcitriol, and the combination therapy resulted in similar reductions in systolic blood pressure. In cultured murine podocytes, ADR stimulated the expression of Bax/Bcl-2 and apoptosis as determined by Hoechst 33342 staining. These changes were effectively inhibited by telmisartan or oxacalcitriol, but the combination treatment most effectively reduced these effects. CONCLUSIONS: These data demonstrated that application of a renin-angiotensin system blocker plus a vitamin D analog effectively prevented renal injury in ADR-induced nephropathy. The observed amelioration of renal injury may be partly attributable to antiapoptotic effects in podocytes.
MADP, a salidroside analog, protects hippocampal neurons from glutamate induced apoptosis.[Pubmed:24631136]
Life Sci. 2014 May 8;103(1):34-40.
AIMS: To investigate the anti-apoptotic effect of MADP, an analog of salidroside, against glutamate induced apoptosis in the cultured rat hippocampal neurons. MAIN METHODS: Cytotoxicity was determined by the MTT method and lactate dehydrogenase release to the medium. Cell apoptosis was evaluated by Hoechst 33342 staining, TUNEL assay and flow cytometric analysis. Western blotting was applied for detecting protein levels of cellular signaling molecules. KEY FINDINGS: Our results showed that glutamate exposure significantly induces cell apoptosis, whereas the pretreatment of salidroside or MADP remarkably improves cell viability. Most importantly, the anti-apoptotic effect of MADP against glutamate insult is superior to salidroside. To explore the involved mechanisms, we measured some pro-apoptotic and anti-apoptotic protein levels, and several cell survival signaling pathways were analyzed as well. No visible alterations in Bcl-2 and Bax protein levels were observed by MADP or salidroside. Akt and JNK phosphorylation was robustly stimulated by MADP in the glutamate-treated neurons. Salidroside treatment results in a slight activation in Akt, while no significant alteration in JNK activity was observed. SIGNIFICANCE: MADP exhibits higher capacity to attenuate glutamate induced cell apoptosis in the cultured rat hippocampal neurons, suggesting that MADP might be a better candidate than salidroside for developing novel drugs treating neuron loss associated disorders.
Pretreatment with 2-(4-methoxyphenyl)ethyl-2-acetamido-2-deoxy-beta-D-pyranoside attenuates cerebral ischemia/reperfusion-induced injury in vitro and in vivo.[Pubmed:24991917]
PLoS One. 2014 Jul 3;9(7):e100126.
Salidroside, extracted from the root of Rhodiola rosea L, is known for its pharmacological properties, in particular its neuroprotective effects. 2-(4-Methoxyphenyl) ethyl-2-acetamido-2-deoxy-beta-D-pyranoside (GlcNAc-Sal), an analog of salidroside, was recently synthesized and shown to possess neuroprotective properties. The purpose of the current study was to investigate the neuroprotective effects of GlcNAc-Sal against oxygen-glucose deprivation-reperfusion (OGD-R)-induced neurotoxicity in vitro and global cerebral ischemia-reperfusion (GCI-R) injury in vivo. Cell viability tests and Hoechst 33342 staining confirmed that GlcNAc-Sal pretreatment markedly attenuated OGD-R induced apoptotic cell death in immortalized mouse hippocampal HT22 cells. Western blot, immunofluorescence and PCR analyses revealed that GlcNAc-Sal pretreatment restored the balance of pro- and anti-apoptotic proteins and inhibited the activation of caspase-3 and PARP induced by OGD-R treatment. Further analyses showed that GlcNAc-Sal pretreatment antagonized reactive oxygen species (ROS) generation, iNOS-derived NO production and NO-related apoptotic cell death during OGD-R stimulation. GCI-R was induced by bilateral common carotid artery occlusion (BCCAO) and reperfusion in mice in vivo. Western blot analysis showed that GlcNAc-Sal pretreatment decreased the expression of caspase-3 and increased the expression of Bcl-2 (B-cell lymphoma 2)/Bax (Bcl-2-associated X protein) induced by GCI-R treatment. Our findings suggest that GlcNAc-Sal pretreatment prevents brain ischemia reperfusion injury by the direct or indirect suppression of cell apoptosis and GlcNAc-Sal could be developed as a broad-spectrum agent for the prevention and/or treatment of cerebral ischemic injury.
Application of JC1 for non-toxic isolation of cells with MDR transporter activity by flow cytometry.[Pubmed:28380010]
PLoS One. 2017 Apr 5;12(4):e0174905.
The DNA intercalating dye Hoechst 33342 or its close analog DCV are actively removed from cells by the multidrug resistance transporter ABCG2, a protein overexpressed in metastatic cells and somatic stem cells. In bivariate blue-red flow cytometry fluorescent plots active Hoechst or DCV efflux combined with a concentration dependent bathochromic shifts of these nuclear dyes leads to the segregation of the transporter-rich cells into a distinct cell cohort tilted towards the shorter wavelength axis of the plot, the cohort is generically known as the side population (SP). This feature has facilitated the surface marker-independent isolation of live stem cells. A drawback, though, is the known toxicity of Hoechst dyes. In this study we show that JC1, a bathochromic mitochondrial membrane potential-sensitive dye applied at proper concentration, can yield flow cytometry fluorescent emission bivariate plots containing a low JC1 accumulation (JC1low) cohort. Using a combination of multiple cell lines, ABC-transporter inhibitors and viral vector-driven insertion of the ABCG2 gene or ABCG2 and ABCB1 shRNAs we demonstrate that JC1low can be generated by either of the two aforementioned multidrug resistance transporters. Complete wash out of mitochondrial bound JC1 required more than 24 h. In spite of this tight binding, the dye did not affect either the mitochondrial membrane potentials or the proliferation rate. In contrast, contemporaneous with its nuclear accumulation, Hoechst 33342 or DVC, caused changes in the fluorescent emission of mitochondrial membrane potential sensitive dyes resembling the effects caused by the mitochondrial uncoupler FCCP. In a number of cell lines exposure to Hoechst resulted in marked slow-down of proliferation and abolition of ABCG2 transport activity during the subsequent 2 days but in K562 cells the exposure induced cell extended death. Overall, its lack of toxicity vis. a vis. the toxicity and genotoxicity of the DNA intercalating dyes makes JC1 an ideal tool for isolating live cells expressing high multidrug resistance transport activity.
The apoptotic pathways in the curcumin analog MHMD-induced lung cancer cell death and the essential role of actin polymerization during apoptosis.[Pubmed:25960227]
Biomed Pharmacother. 2015 Apr;71:128-34.
As a mode of cell death, apoptosis could be triggered by the extrinsic, intrinsic mitochondrial and intrinsic endoplasmic reticulum pathways and actin rearrangement is needed during apoptosis. We previously found that one curcumin analog MHMD could induce A549 lung cancer cells apoptosis. But the apoptotic pathways and the actin dynamics during apoptosis are not known. Here, we detected the activation of caspase-3, -8, -9, -12, PARP and the increase ratio of Bax/Bcl-2 by western blotting in MHMD-exposed A549 cells. Alternatively, caspases inhibitors could lead to the disappearance of MHMD-eliciting nuclei fragmentation by Hoechst 33342 staining. Besides, JC-1 and DCFH-DA staining showed the fall of mitochondrial membrane potential and the release of ROS. Moreover, wound healing assay confirmed the MHMD anti-migration ability, which was much more effective than curcumin. Importantly, unlike other anticarcinogenic drugs, MHMD might induce the actin polymerization but not depolymerization in the process of A549 cell apoptosis by phalloidin-FITC staining, which is essential to MHMD-induced extrinsic, intrinsic mitochondrial and intrinsic ER pathways of cell apoptosis.


