OLDAPotent, selective endogenous TRPV1 agonist CAS# 105955-11-1 |
- Pyridostigmine Bromide
Catalog No.:BCC4579
CAS No.:101-26-8
- MK-0974
Catalog No.:BCC1756
CAS No.:781649-09-0
- MK-3207
Catalog No.:BCC1759
CAS No.:957118-49-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 105955-11-1 | SDF | Download SDF |
| PubChem ID | 5282106 | Appearance | Powder |
| Formula | C26H43NO3 | M.Wt | 417.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | <em>N</em>-Oleoyldopamine | ||
| Solubility | Soluble to 50 mM in DMSO and to 50 mM in ethanol | ||
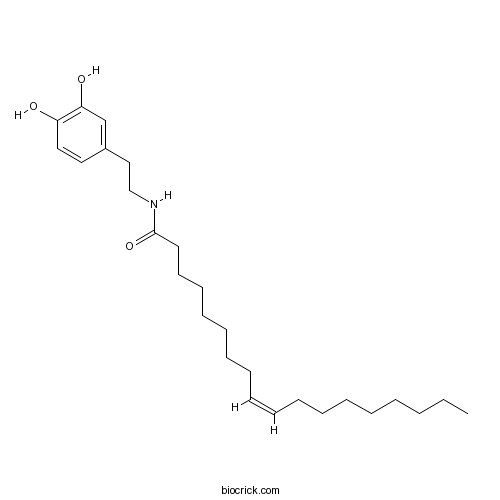
| Chemical Name | (Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]octadec-9-enamide | ||
| SMILES | CCCCCCCCC=CCCCCCCCC(=O)NCCC1=CC(=C(C=C1)O)O | ||
| Standard InChIKey | QQBPLXNESPTPNU-KTKRTIGZSA-N | ||
| Standard InChI | InChI=1S/C26H43NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26(30)27-21-20-23-18-19-24(28)25(29)22-23/h9-10,18-19,22,28-29H,2-8,11-17,20-21H2,1H3,(H,27,30)/b10-9- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent endogenous vanilloid TRPV1 (VR1) receptor agonist (EC50 = 36 nM at hVR1) with low affinity for rCB1 receptors (Ki = 1.6 μM). Potently induces VR1-mediated thermal hyperalgesia in rats in vivo. |

OLDA Dilution Calculator

OLDA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3945 mL | 11.9723 mL | 23.9446 mL | 47.8893 mL | 59.8616 mL |
| 5 mM | 0.4789 mL | 2.3945 mL | 4.7889 mL | 9.5779 mL | 11.9723 mL |
| 10 mM | 0.2394 mL | 1.1972 mL | 2.3945 mL | 4.7889 mL | 5.9862 mL |
| 50 mM | 0.0479 mL | 0.2394 mL | 0.4789 mL | 0.9578 mL | 1.1972 mL |
| 100 mM | 0.0239 mL | 0.1197 mL | 0.2394 mL | 0.4789 mL | 0.5986 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- STEARDA
Catalog No.:BCC7288
CAS No.:105955-10-0
- Doxycycline HCl
Catalog No.:BCC3772
CAS No.:10592-13-9
- Taraxasterol
Catalog No.:BCN5869
CAS No.:1059-14-9
- (tert-Butoxycarbonyl)oxycefcapene pivoxil
Catalog No.:BCC8403
CAS No.:105889-80-3
- Obtusilin
Catalog No.:BCN2697
CAS No.:105870-59-5
- TSTU
Catalog No.:BCC2828
CAS No.:105832-38-0
- Tropisetron Hydrochloride
Catalog No.:BCC4027
CAS No.:105826-92-4
- Nateglinide
Catalog No.:BCC5005
CAS No.:105816-04-4
- E-3810
Catalog No.:BCC1541
CAS No.:1058137-23-7
- Sitostenone
Catalog No.:BCN5868
CAS No.:1058-61-3
- Fmoc-Ser(tBu)-OPfp
Catalog No.:BCC3545
CAS No.:105751-13-1
- Methyl ganoderate C6
Catalog No.:BCN3259
CAS No.:105742-81-2
- Clinafloxacin CI96 AM1091
Catalog No.:BCC3754
CAS No.:105956-97-6
- Sulfocostunolide B
Catalog No.:BCN5870
CAS No.:1059671-65-6
- 2-(3,4-Dihydroxyphenyl)ethanol
Catalog No.:BCN5871
CAS No.:10597-60-1
- Geraniol
Catalog No.:BCN2631
CAS No.:106-24-1
- Nerol
Catalog No.:BCN8517
CAS No.:106-25-2
- β-Interleukin I (163-171), human
Catalog No.:BCC1017
CAS No.:106021-96-9
- Palmatine hydrochloride
Catalog No.:BCN5914
CAS No.:10605-02-4
- Hoechst 33342 analog 2
Catalog No.:BCC1631
CAS No.:106050-84-4
- Senktide
Catalog No.:BCC6921
CAS No.:106128-89-6
- PND-1186
Catalog No.:BCC1866
CAS No.:1061353-68-1
- TC-G 1004
Catalog No.:BCC6165
CAS No.:1061747-72-5
- WAY-600
Catalog No.:BCC4607
CAS No.:1062159-35-6
[Discrimination of pork storage time using near infrared spectroscopy and Adaboost+OLDA].[Pubmed:23427543]
Guang Pu Xue Yu Guang Pu Fen Xi. 2012 Dec;32(12):3238-41.
Pork storage time is closely related to its freshness. With the help of near infrared diffuse reflectance spectroscopy, pork sample data were collected. The orthogonal linear discriminant analysis (OLDA) algorithm was used to extract features. Furthermore, by introducing Adaboost algorithm to OLDA, a new algorithm, named Adaboost+OLDA, was proposed based on OLDA and Adaboost. To investigate the classification rate and the computational time of Adaboost+OLDA algorithm, the classical feature extraction methods (PCA+LDA and OLDA) were compared with Adaboost+OLDA in the experiments. Experimental results showed that Adaboost+OLDA could be computed efficiently and in improved the generalization ability of OLDA. The average classification rate of Adaboost+OLDA is more than 95%.
Complete Genome Sequences of the Historical Legionella pneumophila Strains OLDA and Pontiac.[Pubmed:27563044]
Genome Announc. 2016 Aug 25;4(4). pii: 4/4/e00866-16.
Here, we report the complete genome sequences of Legionella pneumophila serogroup 1 strains OLDA and Pontiac, which predate the 1976 Philadelphia Legionnaires' disease outbreak. Strain OLDA was isolated in 1947 from an apparent sporadic case, and strain Pontiac caused an explosive outbreak at a Michigan health department in 1968.
Antiallodynic effects of loperamide and fentanyl against topical capsaicin-induced allodynia in unanesthetized primates.[Pubmed:15152028]
J Pharmacol Exp Ther. 2004 Oct;311(1):155-63.
Capsaicin produces thermal allodynia in animals and humans by acting as an agonist at vanilloid receptor subtype 1 [VR1; also known as transient receptor potential vanilloid type 1 (TRPV1)]. VR1 receptors are widely distributed in the periphery (e.g., on primary afferent neurons). These studies examined the ability of loperamide (0.1-1 mg/kg s.c.; a micro-opioid agonist that is peripherally selective after systemic administration), in preventing and reversing thermal allodynia caused by topical capsaicin (0.004 M) in rhesus monkeys, within a tail withdrawal assay (n = 4; 38 degrees C and 42 degrees C; normally non-noxious thermal stimuli). The effects of loperamide were compared with those of the centrally penetrating micro-agonist, fentanyl (0.0032-0.032 mg/kg s.c.). We also characterized the allodynic effects of the endogenous VR1 agonist ("endovanilloid"), N-oleoyldopamine (OLDA; 0.0013-0.004 M). In this model, loperamide and fentanyl produced dose-dependent prevention of capsaicin-induced allodynia, whereas only fentanyl produced robust reversal of ongoing allodynia. Antagonism experiments with naltrexone (0.1 mg/kg s.c.) or its analog, methylnaltrexone (0.32 mg/kg s.c.), which does not readily cross the blood-brain barrier, suggest that the antiallodynic effects of loperamide and fentanyl were predominantly mediated by peripherally and centrally located micro-receptors, respectively. Loperamide and fentanyl (1 mg/kg and 0.032 mg/kg, respectively) also prevented OLDA (0.004 M)-induced allodynia. Up to the largest dose studied, loperamide was devoid of thermal antinociceptive effects at 48 degrees C (a noxious thermal stimulus, in the absence of capsaicin). By contrast, fentanyl (0.01-0.032 mg/kg) caused dose-dependent antinociception in this sensitive thermal antinociceptive assay (a presumed centrally mediated effect). These studies show that loperamide, acting as a peripherally selective micro-agonist after systemic administration, can prevent capsaicin-induced thermal allodynia in primates in vivo, in the absence of thermal antinociceptive effects.
N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia.[Pubmed:12569099]
J Biol Chem. 2003 Apr 18;278(16):13633-9.
N-Arachidonoyldopamine (NADA) was recently identified as an endogenous ligand for the vanilloid type 1 receptor (VR1). Further analysis of the bovine striatal extract from which NADA was isolated indicated the existence of substances corresponding in molecular mass to N-oleoyldopamine (OLDA), N-palmitoyldopamine (PALDA), and N-stearoyldopamine (STEARDA). Quadrupole time-of-flight mass spectrometric analysis of bovine striatal extracts revealed the existence of OLDA, PALDA, and STEARDA as endogenous compounds in the mammalian brain. PALDA and STEARDA failed to affect calcium influx in VR1-transfected human embryonic kidney (HEK) 293 cells or paw withdrawal latencies from a radiant heat source, and there was no evidence of spontaneous pain behavior. By contrast, OLDA induced calcium influx (EC(50) = 36 nm), reduced the latency of paw withdrawal from a radiant heat source in a dose-dependent manner (EC(50) = 0.72 microg), and produced nocifensive behavior. These effects were blocked by co-administration of the VR1 antagonist iodo-resiniferatoxin (10 nm for HEK cells and 1 microg/50 micro;l for pain behavior). These findings demonstrate the existence of an endogenous compound in the brain that is similar to capsaicin and NADA in its chemical structure and activity on VR1. Unlike NADA, OLDA was only a weak ligand for rat CB1 receptors; but like NADA, it was recognized by the anandamide membrane transporter while being a poor substrate for fatty-acid amide hydrolase. Analysis of the activity of six additional synthetic and potentially endogenous N-acyldopamine indicated the requirement of a long unsaturated fatty acid chain for an optimal functional interaction with VR1 receptors.


