MK-3207CGRP receptor antagonist CAS# 957118-49-9 |
- Pyridostigmine Bromide
Catalog No.:BCC4579
CAS No.:101-26-8
- Olcegepant
Catalog No.:BCC1818
CAS No.:204697-65-4
- MK-0974
Catalog No.:BCC1756
CAS No.:781649-09-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 957118-49-9 | SDF | Download SDF |
| PubChem ID | 25019940 | Appearance | Powder |
| Formula | C31H29F2N5O3 | M.Wt | 557.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (179.34 mM) *"≥" means soluble, but saturation unknown. | ||
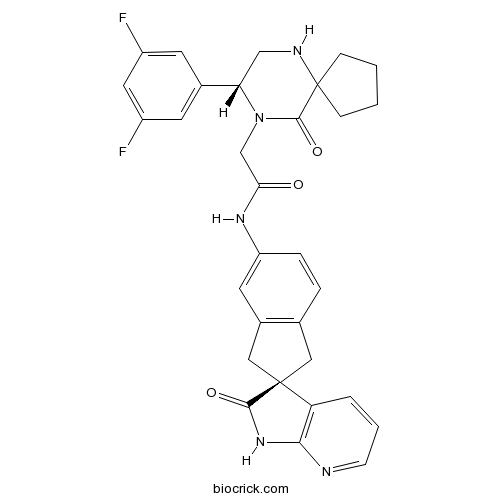
| Chemical Name | 2-[(8R)-8-(3,5-difluorophenyl)-10-oxo-6,9-diazaspiro[4.5]decan-9-yl]-N-[(2R)-2'-oxospiro[1,3-dihydroindene-2,3'-1H-pyrrolo[2,3-b]pyridine]-5-yl]acetamide | ||
| SMILES | C1CCC2(C1)C(=O)N(C(CN2)C3=CC(=CC(=C3)F)F)CC(=O)NC4=CC5=C(CC6(C5)C7=C(NC6=O)N=CC=C7)C=C4 | ||
| Standard InChIKey | AZAANWYREOQRFB-SETSBSEESA-N | ||
| Standard InChI | InChI=1S/C31H29F2N5O3/c32-21-10-19(11-22(33)13-21)25-16-35-31(7-1-2-8-31)29(41)38(25)17-26(39)36-23-6-5-18-14-30(15-20(18)12-23)24-4-3-9-34-27(24)37-28(30)40/h3-6,9-13,25,35H,1-2,7-8,14-17H2,(H,36,39)(H,34,37,40)/t25-,30+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | MK-3207 is a highly potent, orally bioavailable antagonist of CGRP receptor with IC50 value of 0.12 nM. | |||||
| Targets | CGRP | |||||
| IC50 | 0.12 nM | |||||

MK-3207 Dilution Calculator

MK-3207 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7934 mL | 8.9672 mL | 17.9343 mL | 35.8686 mL | 44.8358 mL |
| 5 mM | 0.3587 mL | 1.7934 mL | 3.5869 mL | 7.1737 mL | 8.9672 mL |
| 10 mM | 0.1793 mL | 0.8967 mL | 1.7934 mL | 3.5869 mL | 4.4836 mL |
| 50 mM | 0.0359 mL | 0.1793 mL | 0.3587 mL | 0.7174 mL | 0.8967 mL |
| 100 mM | 0.0179 mL | 0.0897 mL | 0.1793 mL | 0.3587 mL | 0.4484 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MK-3207 is a new, highly selective CGRP receptor antagonist that is approximately 40- to 65-fold more potent than telcagepant for the human CGRP receptor. The IC50 is 0.12 nM and The Ki value is 0.024 nM. It is highly selective versus human AM1, AM2, CTR, and AMY3. [1]
Calcitonin gene-related peptide (CGRP) is a 37 amino acid neuropeptide that is widely distributed in the central and peripheral nervous system. The CGRP receptor is composed of the calcitonin receptor-like receptor (CLR), a family B G protein-coupled receptor (GPCR), in association with receptor activity modifying protein 1 (RAMP1) and receptor component protein. A number of lines of evidence implicated CGRP in migraine pathophysiology, and this led to interest in the potential utility of CGRP receptor antagonists as novel therapeutics for migraine. [2]
MK-3207 displayed high affinity for the native human CGRP receptor in SK-N-MC cells and for the recombinant human receptor as measured by the ability to compete with 125I-hCGRP binding, with Ki values of 0.024 ± 0.001 nM and 0.022 ± 0.002 nM, respectively. MK-3207 displayed a similar affinity (Ki) for the rhesus monkey receptor (0.024 ± 0.001 nM) as for human, but it displayed 400-fold lower affinity for the canine and rat receptors, with values of 10 nM and 10 ± 1.2 nM , respectively. MK-3207 potently blocked human α-CGRP-stimulated cAMP responses in human CGRP receptor-expressing HEK293 cells, with an IC50 value of 0.12 ±0.02 nM . Addition of 50% human serum (IC50 =0.17 ± 0.02nM) had little effect on the apparent potency ofMK-3207. [3]
MK-3207 has EC50 and Emax values of approximately 0.8 ± 0.3 nM (mean ± S.E.) and 81 ± 5% (mean ± S.E.), respectively, for inhibition of CIDV in rhesus monkeys. The expected EC90 value in rhesus monkey is therefore approximately 7 nM (9-fold higher than the estimated EC50 value). After an oral dose of 10 mg/kg MK-3207, the CSF/plasma ratio is 2 to 3% . However, the CSF/ plasma ratio is approximately 30% of the unbound fraction (9.4%) in plasma, indicating that the central and peripheral compartments are not freely equilibrating.
References:
[1] David J Hewitt, Sheena K Aurora, David W Dodick et al. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia 31(6) 712–722.
[2] Ian M. Bell, Steven N. Gallicchio, Michael R. Wood et al. Discovery of MK-3207: A Highly Potent, Orally Bioavailable CGRP Receptor Antagonist. ACS Med. Chem. Lett. 2010, 1, 24–29.
[3] Christopher A. Salvatore, Eric L. Moore, Amy Calamari, Jacquelynn J. Cook, et al. Pharmacological Properties of MK-3207, a Potent and Orally Active Calcitonin Gene-Related Peptide Receptor Antagonist. doi:10.1124/jpet.109.163816.
- MK-3207 HCl
Catalog No.:BCC4420
CAS No.:957116-20-0
- GDC-0941 dimethanesulfonate
Catalog No.:BCC1590
CAS No.:957054-33-0
- GDC-0941
Catalog No.:BCC3626
CAS No.:957054-30-7
- 7-Aminocephalosporanic acid
Catalog No.:BCC4617
CAS No.:957-68-6
- Isolinderalactone
Catalog No.:BCN1252
CAS No.:957-66-4
- XL147
Catalog No.:BCC2487
CAS No.:956958-53-5
- PF-04217903 methanesulfonate
Catalog No.:BCC1849
CAS No.:956906-93-7
- PF-04217903
Catalog No.:BCC2486
CAS No.:956905-27-4
- Betamethasone hydrochloride
Catalog No.:BCC4256
CAS No.:956901-32-9
- Euscaphin B
Catalog No.:BCN4507
CAS No.:956869-95-7
- 8beta,9alpha-Dihydroxylindan-4(5),7(11)-dien-8alpha,12-olide
Catalog No.:BCN8024
CAS No.:956707-04-3
- LDE225 (NVP-LDE225,Erismodegib)
Catalog No.:BCC5066
CAS No.:956697-53-3
- SM-164
Catalog No.:BCC4002
CAS No.:957135-43-2
- NVP-QAV-572
Catalog No.:BCC4181
CAS No.:957209-68-6
- BTZ043 Racemate
Catalog No.:BCC2488
CAS No.:957217-65-1
- Hedyotisol A
Catalog No.:BCN4508
CAS No.:95732-59-5
- Daphnodorin B
Catalog No.:BCN7937
CAS No.:95733-02-1
- Nedaplatin
Catalog No.:BCC4807
CAS No.:95734-82-0
- FPH2 (BRD-9424)
Catalog No.:BCC5451
CAS No.:957485-64-2
- Charybdotoxin
Catalog No.:BCC6933
CAS No.:95751-30-7
- Fmoc-Phe(4-NO2)-OH
Catalog No.:BCC3277
CAS No.:95753-55-2
- Fmoc-Phe(4-NH2)-OH
Catalog No.:BCC3154
CAS No.:95753-56-3
- HIV-1 integrase inhibitor 2
Catalog No.:BCC1619
CAS No.:957890-42-5
- MPC-3100
Catalog No.:BCC2128
CAS No.:958025-66-6
Discovery of MK-3207: A Highly Potent, Orally Bioavailable CGRP Receptor Antagonist.[Pubmed:24900170]
ACS Med Chem Lett. 2010 Jan 12;1(1):24-9.
Incorporation of polar functionality into a series of highly potent calcitonin gene-related peptide (CGRP) receptor antagonists was explored in an effort to improve pharmacokinetics. This strategy identified piperazinone analogues that possessed improved solubility at acidic pH and increased oral bioavailability in monkeys. Further optimization led to the discovery of the clinical candidate 2-[(8R)-8-(3,5-difluorophenyl)-10-oxo-6,9-diazaspiro[4.5]dec-9-yl]-N-[(2R)-2'-oxo -1,1',2',3-tetrahydrospiro[indene-2,3'-pyrrolo[2,3-b]pyridin]-5-yl]acetamide (MK-3207) (4), the most potent orally active CGRP receptor antagonist described to date.
Characterizing the PK/PD relationship for inhibition of capsaicin-induced dermal vasodilatation by MK-3207, an oral calcitonin gene related peptide receptor antagonist.[Pubmed:25377933]
Br J Clin Pharmacol. 2015 May;79(5):831-7.
AIMS: Calcitonin gene related peptide (CGRP) receptor antagonists are effective acute migraine treatments. A capsaicin-induced dermal vasodilatation (CIDV) model has been developed to provide target-engagement information in healthy volunteers. In the model, CGRP release is provoked after dermal capsaicin application, by activating transient receptor potential vanilloid-type-1 (TRPV1) receptors at peripheral sensory nerves. Laser Doppler imaging is used to quantify CIDV and subsequent inhibition by CGRP receptor antagonists. We sought to evaluate a CGRP receptor antagonist, MK-3207, in the biomarker model and to assess the predictability of the CIDV response to migraine clinical efficacy. METHODS: An integrated population pharmacokinetic/pharmacodynamic (PK/PD) model was developed to describe the exposure-response relationship for CIDV inhibition by CGRP and TRPV1 receptor antagonists. MK-3207 dose-response predictions were made based on estimated potency from the PK/PD model and mean plasma concentrations observed at the doses investigated. RESULTS: The results suggested that a 20 mg dose of MK-3207 (EC50 of 1.59 nm) would be required to attain the peripheral CIDV response at a target level that was shown previously to correlate with 2 h clinical efficacy based on phase 3 telcagepant clinical data, and that a plateau of the dose-response would be reached around 40-100 mg. These predictions provided a quantitative rationale for dose selection in a phase 2 clinical trial of MK-3207 and helped with interpretation of the efficacy results from the trial. CONCLUSIONS: The integrated CIDV PK/PD model provides a useful platform for characterization of PK/PD relationships and predictions of dose-response relationships to aid in future development of CGRP and TRPV1 receptor antagonists.
Pharmacological properties of MK-3207, a potent and orally active calcitonin gene-related peptide receptor antagonist.[Pubmed:20065019]
J Pharmacol Exp Ther. 2010 Apr;333(1):152-60.
Calcitonin gene-related peptide (CGRP) has long been hypothesized to play a key role in migraine pathophysiology, and the advent of small-molecule antagonists has clearly demonstrated a clinical link between blocking the CGRP receptor and migraine efficacy. 2-[(8R)-8-(3,5-Difluorophenyl)-10-oxo-6,9-diazaspiro[4.5]dec-9-yl]-N-[(2R)-2'-oxo -1,1',2',3-tetrahydrospiro[indene-2,3'-pyrrolo[2,3-b]pyridin]-5-yl]acetamide (MK-3207) represents the third CGRP receptor antagonist to display clinical efficacy in migraine trials. Here, we report the pharmacological characterization of MK-3207, a potent and orally bioavailable CGRP receptor antagonist. In vitro, MK-3207 is a potent antagonist of the human and rhesus monkey CGRP receptors (K(i) = 0.024 nM). In common with other CGRP receptor antagonists, MK-3207 displays lower affinity for CGRP receptors from other species, including canine and rodent. As a consequence of species selectivity, the in vivo potency was assessed in a rhesus monkey pharmacodynamic assay measuring capsaicin-induced changes in forearm dermal blood flow via laser Doppler imaging. MK-3207 produced a concentration-dependent inhibition of dermal vasodilation, with plasma concentrations of 0.8 and 7 nM required to block 50 and 90% of the blood flow increase, respectively. The tritiated analog [3H]MK-3207 was used to study the binding characteristics on the human CGRP receptor. [3H]MK-3207 displayed reversible and saturable binding (K(D) = 0.06 nM), and the off-rate was determined to be 0.012 min(-1), with a t(1/2) value of 59 min. In vitro autoradiography studies on rhesus monkey brain slices identified the highest level of binding in the cerebellum, brainstem, and meninges. Finally, as an index of central nervous system penetrability, the in vivo cerebrospinal fluid/plasma ratio was determined to be 2 to 3% in cisterna magna-ported rhesus monkeys.
Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine.[Pubmed:21383045]
Cephalalgia. 2011 Apr;31(6):712-22.
BACKGROUND: This study evaluated the CGRP receptor antagonist MK-3207 for acute treatment of migraine. METHODS: Multicenter, double-blind, randomized, placebo-controlled, parallel-group, two-stage adaptive study with two interim efficacy analyses to facilitate optimal dose selection. Migraine patients were initially randomized to MK-3207 2.5, 5, 10, 20, 50 and 100 mg or placebo to treat a moderate/severe migraine. One or more doses were to be discontinued based on the first interim analysis and a lower or higher dose could be added based on the second interim analysis. The primary endpoint was two-hour pain freedom. RESULTS: A total of 547 patients took study medication. After the first interim analysis, the two lowest MK-3207 doses (2.5, 5 mg) were identified as showing insufficient efficacy. Per the pre-specified adaptive design decision rule, only the 2.5-mg group was discontinued and the five highest doses (5, 10, 20, 50, 100 mg) were continued into the second stage. After the second interim efficacy analysis, a 200 mg dose was added due to insufficient efficacy at the top three (20, 50, 100 mg) doses. A positive dose-response trend was demonstrated when data were combined across all MK-3207 doses for two-hour pain freedom (p < .001). The pairwise difference versus placebo for two-hour pain freedom was significant for 200 mg (p < .001) and nominally significant for 100 mg and 10 mg (p < .05). The incidence of adverse events appeared comparable between active treatment groups and placebo, and did not appear to increase with increasing dose. CONCLUSIONS: MK-3207 was effective and generally well tolerated in the acute treatment of migraine.


