GDC-0941 dimethanesulfonatePI3K inhibitor CAS# 957054-33-0 |
- AZD6244 (Selumetinib)
Catalog No.:BCC3624
CAS No.:606143-52-6
- Trametinib (GSK1120212)
Catalog No.:BCC1282
CAS No.:871700-17-3
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 957054-33-0 | SDF | Download SDF |
| PubChem ID | 56972143 | Appearance | Powder |
| Formula | C25H35N7O9S4 | M.Wt | 705.85 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Pictilisib dimethanesulfonate; GDC-0941 (2 MeSO3H salt); GDC-0941 | ||
| Solubility | DMSO : 50 mg/mL (70.84 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
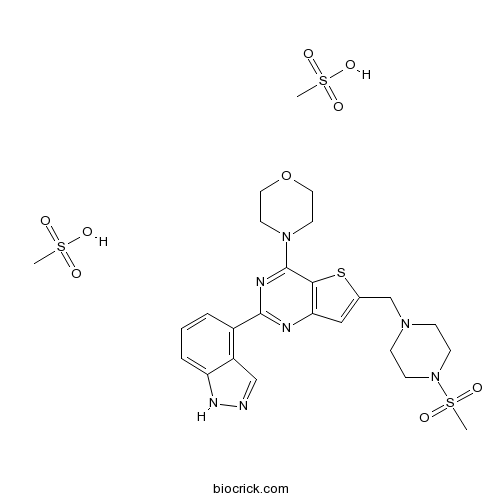
| Chemical Name | 4-[2-(1H-indazol-4-yl)-6-[(4-methylsulfonylpiperazin-1-yl)methyl]thieno[3,2-d]pyrimidin-4-yl]morpholine;methanesulfonic acid | ||
| SMILES | CS(=O)(=O)N1CCN(CC1)CC2=CC3=C(S2)C(=NC(=N3)C4=C5C=NNC5=CC=C4)N6CCOCC6.CS(=O)(=O)O.CS(=O)(=O)O | ||
| Standard InChIKey | RFRIKACSFOTIMU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H27N7O3S2.2CH4O3S/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19;2*1-5(2,3)4/h2-4,13-14H,5-12,15H2,1H3,(H,24,27);2*1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GDC-0941 dimethanesulfonate is an orally bioavailable and selective inhibitor of class I PI3K with IC50 values of 15 nM, 185 nM, 7 nM and 224 nM for PI3Kα, PI3Kβ, PI3Kδ and PI3Kγ, respectively. | ||||||
| Targets | PI3Kα | PI3Kβ | PI3Kδ | PI3Kγ | |||
| IC50 | 15 nM | 185 nM | 7 nM | 224 nM | |||
| Cell experiment: [1] | |
| Cell lines | MDA-MB-231 and SKBR3 cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 10 μM for MDA-MB-231 cells 1.25 μM for SKBR3 cells 72 hours |
| Applications | The cytotoxicity was determined using MTT assay, after 72 h exposure to GDC-0941, ABT-737 and the combination. 10-times greater concentration of ABT-737 as a single agent resulted in a less than 50% increase in cytotoxicity. Regardless of the sensitivity to single agents, the combination of GDC-0941 and ABT-737 induced a more significant reduction of viable cells, indicating potent synergistic effect of GDC-0941 and ABT-737. |
| Animal experiment: [2] | |
| Animal models | Female severe combined immunodeficient mice injected with breast cancer cells |
| Dosage form | Oral administration, 100 or 150 mg/kg |
| Application | GDC-0941 showed excellent antitumor activity in xenograft models with HER2 amplification, PIK3CA mutations, or concomitant alterations in two pathway components (e.g., PTEN loss and PIK3CA mutation), but little or no effect in xenografts of basal-like KRAS mutant MDA-MB-231 cells. Treatment with GDC-0941 at a dose of 100 mg/kg substantially down-regulated levels of pAKT(S473) in xenograft tumors of both sensitive KPL-4 cells and resistant MDA-MB-231 cells after 1 hour, suggesting effective pharmacodynamic inhibition of PI3K signaling at this dose in both models. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Zheng L, Yang W, Zhang C, et al. GDC-0941 sensitizes breast cancer to ABT-737 in vitro and in vivo through promoting the degradation of Mcl-1. Cancer letters, 2011, 309(1): 27-36. [2] O'Brien C, Wallin J J, Sampath D, et al. Predictive biomarkers of sensitivity to the phosphatidylinositol 3′ kinase inhibitor GDC-0941 in breast cancer preclinical models. Clinical Cancer Research, 2010, 16(14): 3670-3683. | |

GDC-0941 dimethanesulfonate Dilution Calculator

GDC-0941 dimethanesulfonate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4167 mL | 7.0837 mL | 14.1673 mL | 28.3346 mL | 35.4183 mL |
| 5 mM | 0.2833 mL | 1.4167 mL | 2.8335 mL | 5.6669 mL | 7.0837 mL |
| 10 mM | 0.1417 mL | 0.7084 mL | 1.4167 mL | 2.8335 mL | 3.5418 mL |
| 50 mM | 0.0283 mL | 0.1417 mL | 0.2833 mL | 0.5667 mL | 0.7084 mL |
| 100 mM | 0.0142 mL | 0.0708 mL | 0.1417 mL | 0.2833 mL | 0.3542 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GDC-0941 is a novel selective class I phosphatidylinositol-3-kinase (PI3K) inhibitor. Activation of PI3K/Akt signaling pathway is frequently associated with tumorigenesis. Deregulation of this pathway occurs frequently with a variety of cancers and may contribute to the resistance to many anticancer agents. [1] Developing novel small molecules that specifically block the PI3K/Akt pathway may inhibit tumor growth. GDC-0941 is designed to bind the ATP-binding pocket of PI3K and to prevent formation of phosphatidylinositol-3, 4, 5-triphosphate (PIP3), a second messenger that transmits PI3K downstream signals. [2, 3] It binds to PI3K in an ATP-competitive manner.
GDC-0941 is a potent small-molecule thieno [3, 2-d] pyrimidine inhibitor of the class I PI3K. It is highly selective against isoforms p110( and p110( with IC50 of 3 nM, and moderately selective against isoforms p110( and p110( with IC50s of 33 nM and 75 nM, respectively.
GDC-0941 inhibits cell proliferation in vitro and in vivo. It causes growth inhibition in a variety of cancer cell lines, including A2780, MDA-MB-361, PC3, and U87MG. [2] It also inhibits the growth of trastuzumab–sensitive and –resistant HER2-amplied cancer cells which harbor p110( mutations or PTEN loss. [4] GDC-0941 also reduces tumor volume in different xenograft models. [4]
GDC-0941 can be taken orally.
References:
[1]Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497-5510.
[2]Folkes AJ, Ahmadi K, Alderton WK, et al. The identification of 2-(1H-Indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008; 51: 5522-5532.
[3]Knight ZA, Shokat KM. Chemically targeting the PI3K family. Biochem Soc Trans. 2007; 35: 245-249.
[4]Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Br J Cancer. 2011; 104(7): 1116-25.
- GDC-0941
Catalog No.:BCC3626
CAS No.:957054-30-7
- 7-Aminocephalosporanic acid
Catalog No.:BCC4617
CAS No.:957-68-6
- Isolinderalactone
Catalog No.:BCN1252
CAS No.:957-66-4
- XL147
Catalog No.:BCC2487
CAS No.:956958-53-5
- PF-04217903 methanesulfonate
Catalog No.:BCC1849
CAS No.:956906-93-7
- PF-04217903
Catalog No.:BCC2486
CAS No.:956905-27-4
- Betamethasone hydrochloride
Catalog No.:BCC4256
CAS No.:956901-32-9
- Euscaphin B
Catalog No.:BCN4507
CAS No.:956869-95-7
- 8beta,9alpha-Dihydroxylindan-4(5),7(11)-dien-8alpha,12-olide
Catalog No.:BCN8024
CAS No.:956707-04-3
- LDE225 (NVP-LDE225,Erismodegib)
Catalog No.:BCC5066
CAS No.:956697-53-3
- MM-22
Catalog No.:BCC6114
CAS No.:956605-71-3
- UNBS 5162
Catalog No.:BCC4008
CAS No.:956590-23-1
- MK-3207 HCl
Catalog No.:BCC4420
CAS No.:957116-20-0
- MK-3207
Catalog No.:BCC1759
CAS No.:957118-49-9
- SM-164
Catalog No.:BCC4002
CAS No.:957135-43-2
- NVP-QAV-572
Catalog No.:BCC4181
CAS No.:957209-68-6
- BTZ043 Racemate
Catalog No.:BCC2488
CAS No.:957217-65-1
- Hedyotisol A
Catalog No.:BCN4508
CAS No.:95732-59-5
- Daphnodorin B
Catalog No.:BCN7937
CAS No.:95733-02-1
- Nedaplatin
Catalog No.:BCC4807
CAS No.:95734-82-0
- FPH2 (BRD-9424)
Catalog No.:BCC5451
CAS No.:957485-64-2
- Charybdotoxin
Catalog No.:BCC6933
CAS No.:95751-30-7
- Fmoc-Phe(4-NO2)-OH
Catalog No.:BCC3277
CAS No.:95753-55-2
- Fmoc-Phe(4-NH2)-OH
Catalog No.:BCC3154
CAS No.:95753-56-3


