XL147PI3K inhibitor,selective and reversible CAS# 956958-53-5 |
- CYT387 sulfate salt
Catalog No.:BCC1506
CAS No.:1056636-06-6
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- JAK2 Inhibitor V, Z3
Catalog No.:BCC1667
CAS No.:195371-52-9
- Bardoxolone methyl
Catalog No.:BCC1400
CAS No.:218600-53-4
- Ruxolitinib (INCB018424)
Catalog No.:BCC1276
CAS No.:941678-49-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 956958-53-5 | SDF | Download SDF |
| PubChem ID | 1893730 | Appearance | Powder |
| Formula | C21H16N6O2S2 | M.Wt | 448.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (111.48 mM) *"≥" means soluble, but saturation unknown. | ||
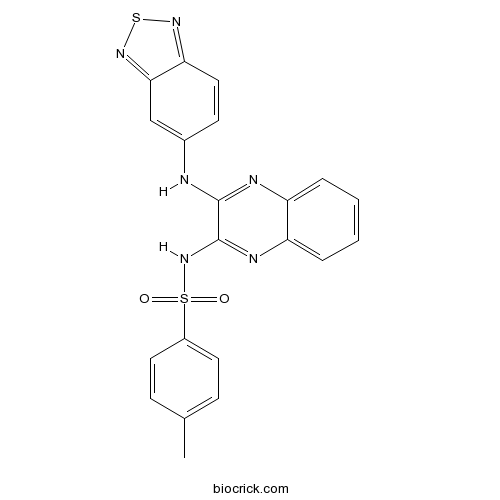
| Chemical Name | N-[3-(2,1,3-benzothiadiazol-5-ylamino)quinoxalin-2-yl]-4-methylbenzenesulfonamide | ||
| SMILES | CC1=CC=C(C=C1)S(=O)(=O)NC2=NC3=CC=CC=C3N=C2NC4=CC5=NSN=C5C=C4 | ||
| Standard InChIKey | MQMKRQLTIWPEDM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H16N6O2S2/c1-13-6-9-15(10-7-13)31(28,29)27-21-20(23-16-4-2-3-5-17(16)24-21)22-14-8-11-18-19(12-14)26-30-25-18/h2-12H,1H3,(H,22,23)(H,24,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | XL147 is a selective and reversible inhibitor of PI3Kα/ PI3Kδ/ PI3Kγ with IC50 values of 39 nM/36 nM/23 nM, respectively. | ||||||
| Targets | PI3Kα | PI3Kδ | PI3Kγ | PI3Kβ | |||
| IC50 | 39 nM | 36 nM | 23 nM | 383 nM | |||

XL147 Dilution Calculator

XL147 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2296 mL | 11.1478 mL | 22.2955 mL | 44.5911 mL | 55.7389 mL |
| 5 mM | 0.4459 mL | 2.2296 mL | 4.4591 mL | 8.9182 mL | 11.1478 mL |
| 10 mM | 0.223 mL | 1.1148 mL | 2.2296 mL | 4.4591 mL | 5.5739 mL |
| 50 mM | 0.0446 mL | 0.223 mL | 0.4459 mL | 0.8918 mL | 1.1148 mL |
| 100 mM | 0.0223 mL | 0.1115 mL | 0.223 mL | 0.4459 mL | 0.5574 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
XL147 is a potent and orally active inhibitor that targets Class I PI3Ks with IC50 values in the nanomolar level. It has PI3K inhibition activities with IC50 of 39,383,36, and 23nM for PI3Kα, PI3Kβ, PI3Kδ,PI3Kγ, respectively.[1]
PI3Ks are a family of enzymes, which phosphorylate the 3'- OH position of the inositol ring of phosphoinositides.PI3Ks are divided into three classes based on structural features and in vitro lipid substrate specificity. The three class-Ia PI3K (p110 α / β / δ ) and the sole class-Ib PI3K (p110 γ ) couple growth factor receptors and G-protein-coupled receptors, respectively, to a wide range of downstream pathways. Signal transduction via the PI3K/Akt pathway is essential for regulating cellular functions, including proliferation, survival, migration, motility and tumorigenesis, in a variety of cell types. XL147 is a reversible ATP-competitive inhibitor, yet is highly selective against over 130 human protein kinases.[1]
MCF7 and PC-3 cell lines were treated with XL147 led to a reduction in the levels of phosphatidylinositol-3,4,5-tris-phosphate (PIP3) and decline in phosphorylation of AKT and ribosomal S6 protein, which are two downstream effectors of PI3K signaling. Furthermore, XL147 demonstrates potent anti-angiogenic effects in tubule formation driven by vascular endothelial growth factor and potent inhibition of cell migration stimulated by hepatocyte growth factor . [1]
Oral administration of XL147 results in eminent inhibition of tumor growth in mice bearing xenografts in which PI3K signaling is activated, including the PTEN-deficient PC-3 prostate adenocarcinoma and MDA-MB-468 breast adenocarcinoma models, and the K-Ras activated Calu-6 non-small cell lung carcinoma model. These effects on pathway signaling correlate with inhibiting tumor cell proliferation, inhibiting tumor angiogenesis, and inducing apoptosis as determined by immunohistochemical analysis. Moreover, combining XL147 in these models with other mTOR/Raptor inhibitors, such as the chemotherapeutic agents paclitaxel and carboplatin, results in enhanced anti-tumor efficacy associated with a substantial increase in apoptosis when compared to the individual agents alone. [2]
References:
1.Shapiro G, Edelman G, Calvo E, et al. Targeting aberrant PI3K pathway signaling with XL147, a potent, selective, and orally bioavailable PI3K inhibitor[J]. Proc 97th Annu Meet AACR, 2007: 14-18.
2.Traynor A M, Kurzrock R, Bailey H H, et al. A phase I safety and pharmacokinetic (PK) study of the PI3K inhibitor XL147 (SAR245408) in combination with paclitaxel (P) and carboplatin (C) in patients (pts) with advanced solid tumors[J]. J Clin Oncol, 2010, 28(15s): 3078.
- PF-04217903 methanesulfonate
Catalog No.:BCC1849
CAS No.:956906-93-7
- PF-04217903
Catalog No.:BCC2486
CAS No.:956905-27-4
- Betamethasone hydrochloride
Catalog No.:BCC4256
CAS No.:956901-32-9
- Euscaphin B
Catalog No.:BCN4507
CAS No.:956869-95-7
- 8beta,9alpha-Dihydroxylindan-4(5),7(11)-dien-8alpha,12-olide
Catalog No.:BCN8024
CAS No.:956707-04-3
- LDE225 (NVP-LDE225,Erismodegib)
Catalog No.:BCC5066
CAS No.:956697-53-3
- MM-22
Catalog No.:BCC6114
CAS No.:956605-71-3
- UNBS 5162
Catalog No.:BCC4008
CAS No.:956590-23-1
- Demethylsonchifolin
Catalog No.:BCN4551
CAS No.:956384-55-7
- Ranolazine 2HCl
Catalog No.:BCC2503
CAS No.:95635-56-6
- Ranolazine
Catalog No.:BCC3847
CAS No.:95635-55-5
- Phoyunnanin C
Catalog No.:BCN3686
CAS No.:956344-38-0
- Isolinderalactone
Catalog No.:BCN1252
CAS No.:957-66-4
- 7-Aminocephalosporanic acid
Catalog No.:BCC4617
CAS No.:957-68-6
- GDC-0941
Catalog No.:BCC3626
CAS No.:957054-30-7
- GDC-0941 dimethanesulfonate
Catalog No.:BCC1590
CAS No.:957054-33-0
- MK-3207 HCl
Catalog No.:BCC4420
CAS No.:957116-20-0
- MK-3207
Catalog No.:BCC1759
CAS No.:957118-49-9
- SM-164
Catalog No.:BCC4002
CAS No.:957135-43-2
- NVP-QAV-572
Catalog No.:BCC4181
CAS No.:957209-68-6
- BTZ043 Racemate
Catalog No.:BCC2488
CAS No.:957217-65-1
- Hedyotisol A
Catalog No.:BCN4508
CAS No.:95732-59-5
- Daphnodorin B
Catalog No.:BCN7937
CAS No.:95733-02-1
- Nedaplatin
Catalog No.:BCC4807
CAS No.:95734-82-0
Phase I Dose-Escalation Study of Pilaralisib (SAR245408, XL147) in Combination with Paclitaxel and Carboplatin in Patients with Solid Tumors.[Pubmed:28275119]
Oncologist. 2017 Apr;22(4):377-e37.
LESSONS LEARNED: Despite involvement of PI3K pathway activation in tumorigenesis of solid tumors, single-agent PI3K inhibitors have shown modest clinical activity.Preclinical evidence suggests that combining PI3K pathway inhibitors and chemotherapy can enhance antitumor effects.In patients with solid tumors, the PI3K inhibitor pilaralisib had a favorable safety profile but did not enhance the antitumor activity of paclitaxel plus carboplatin.Further clinical evaluation is warranted to identify effective combination strategies with PI3K pathway inhibitors. BACKGROUND: Pilaralisib (SAR245408) is an oral, pan-class I phosphoinositide 3-kinase (PI3K) inhibitor. This phase I dose-escalation study evaluated the maximum tolerated dose (MTD), safety, pharmacokinetics (PK), and pharmacodynamics of pilaralisib in capsule and tablet formulations, administered in combination with paclitaxel and carboplatin in patients with advanced solid tumors. METHODS: A 3 + 3 design was used. Pilaralisib was administered once daily (QD); paclitaxel (up to 175 mg/m(2)) and carboplatin (up to area under the curve [AUC] of 6) were administered on day 1 of 21-day cycles. An MTD expansion cohort of patients with endometrial carcinoma was included. RESULTS: Fifty-eight patients were enrolled. Six patients (10.3%) had dose-limiting toxicities, of which only rash (two patients, 3.4%) occurred in more than one patient. The MTD of pilaralisib tablets in combination with paclitaxel and carboplatin was determined to be 200 mg QD. The most frequently reported adverse events (AEs) of any grade were neutropenia (67.2%) and thrombocytopenia (67.2%). PK data showed no interaction between pilaralisib and paclitaxel/carboplatin. Tumor tissue showed moderate inhibition of PI3K and mitogen-activated protein kinase (MAPK) pathways. Seven of 52 evaluable patients had a partial response (PR; 13.5%). CONCLUSION: Pilaralisib had a favorable safety profile but did not enhance the antitumor activity of paclitaxel plus carboplatin in solid tumors. The Oncologist 2017;22:377-378.
Dual PI3K/mTOR inhibitor, XL765 (SAR245409), shows superior effects to sole PI3K [XL147 (SAR245408)] or mTOR [rapamycin] inhibition in prostate cancer cell models.[Pubmed:26219891]
Tumour Biol. 2016 Jan;37(1):341-51.
Deregulation of phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathway contributes to prostate cancer development and progression. Here, we compared the in vitro effects of the dual PI3K/mTOR inhibitor (XL765) with those observed with the sole PI3K (XL147) or mTOR (rapamycin) inhibition in 2 non-tumor prostate epithelial cell lines, 8 prostate cancer cell lines, and 11 prostate cancer cell derivatives. We demonstrated that the XL765 treatment showed superior and proliferative effects of XL147 or rapamycin. The XL765 effects were associated to increasing the chromosome region maintenance 1 (CRM1)-mediated nuclear localization of glycogen synthase kinase 3 beta (GSK3beta) and Foxo-1a with higher induction of apoptosis when compared to those observed in XL147 and rapamycin treatments. IC50 values were calculated in phosphatase and tensin homologue deleted on chromosome 10 (PTEN)-positive and PTEN-negative cell lines as well as after PTEN transfection or PTEN downmodulation by siRNA strategy revealing that the presence of this protein was associated with reduced sensitivity to PI3K and mTOR inhibitors. The comparison of IC50 values was also calculated for androgen-dependent and -independent cell lines as well as after androgen receptor (AR) transfection or the AR downmodulation by siRNA strategy revealing that androgen independence was associated with enhanced responsiveness. Our results provide a rationale to use the dual PI3K/Akt/mTOR inhibitors in hormone-insensitive prostate cancer models due to the overactivity of PI3K/Akt/mTOR in this disease condition.
Phase I Trial of the Pan-PI3K Inhibitor Pilaralisib (SAR245408/XL147) in Patients with Chronic Lymphocytic Leukemia (CLL) or Relapsed/Refractory Lymphoma.[Pubmed:25840972]
Clin Cancer Res. 2015 Jul 15;21(14):3160-9.
PURPOSE: This phase I expansion-cohort study evaluated the safety, pharmacokinetics, pharmacodynamics, and preliminary efficacy of the pan-PI3K inhibitor pilaralisib (SAR245408/XL147) in patients with chronic lymphocytic leukemia (CLL) or relapsed or refractory lymphoma. PATIENTS AND METHODS: Patients were treated with the maximum tolerated dose of pilaralisib previously determined in patients with solid tumors (600 mg capsules once daily). Adverse events (AE) and response were evaluated. Plasma pharmacokinetics and pharmacodynamic effects on cytokines and chemokines were also assessed. RESULTS: Twenty-five patients were included in the study: 10 with CLL and 15 with lymphoma. The most frequent AEs of any grade were diarrhea (92.0%), pyrexia (52.0%), and fatigue (44.0%). The most frequent grade >/=3 AEs were neutropenia (32.0%), diarrhea (20.0%), and anemia (16.0%). Pilaralisib exposure on cycle 1 day 28 was similar to exposure in patients with solid tumors. In patients with CLL, pilaralisib significantly reduced plasma levels of several cytokines and chemokines involved in B-cell trafficking. Five patients (50.0%) with CLL and 3 patients (20.0%) with lymphoma had a partial response. Six patients (60.0%) with CLL had nodal shrinkage >/=50%. Overall, 14 patients (56.0%; 7 patients with CLL and 7 patients with lymphoma) had progression-free survival >/=6 months. CONCLUSIONS: Pilaralisib demonstrated an acceptable safety profile in patients with CLL and lymphoma, generally consistent with findings in patients with solid tumors. Single-agent pilaralisib showed preliminary clinical activity in patients with CLL and lymphoma, supporting further development.


