STEARDAEndogenous inhibitor of 5-lipoxygenase CAS# 105955-10-0 |
- Fasudil
Catalog No.:BCC5262
CAS No.:103745-39-7
- Ro 31-8220
Catalog No.:BCC4295
CAS No.:125314-64-9
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- Sotrastaurin (AEB071)
Catalog No.:BCC3857
CAS No.:425637-18-9
- Saikosaponin B2
Catalog No.:BCN5916
CAS No.:58316-41-9
- K-252c
Catalog No.:BCC3706
CAS No.:85753-43-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 105955-10-0 | SDF | Download SDF |
| PubChem ID | 10025103 | Appearance | Powder |
| Formula | C26H45NO3 | M.Wt | 419.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
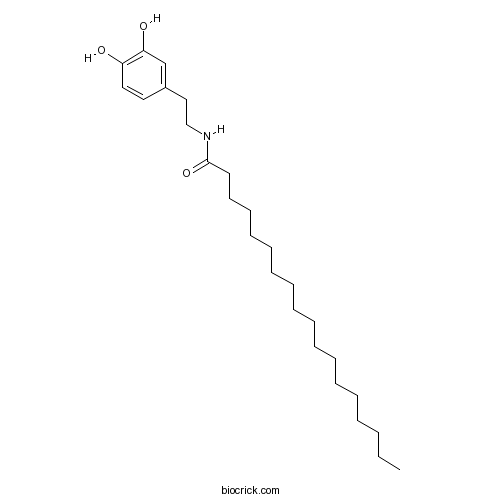
| Synonyms | <em>N</em>-Stearoyldopamine | ||
| Solubility | Soluble to 5 mM in ethanol with gentle warming | ||
| Chemical Name | N-[2-(3,4-dihydroxyphenyl)ethyl]octadecanamide | ||
| SMILES | CCCCCCCCCCCCCCCCCC(=O)NCCC1=CC(=C(C=C1)O)O | ||
| Standard InChIKey | KOCSVLPLQCBIGW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H45NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26(30)27-21-20-23-18-19-24(28)25(29)22-23/h18-19,22,28-29H,2-17,20-21H2,1H3,(H,27,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous fatty acid dopamide that displays 'entourage' effects on endovanilloids NADA and anandamide. Inactive at TRPV1 and CB1 receptors (at concentrations up to 5 μM) and does not inhibit AMT or FAAH (IC50 > 25 μM). However, potentiates TRPV1-mediated effects of NADA in vitro and in vivo; enhances effects on intracellular Ca2+ (EC50 lowered 3-fold) and nociception. Also inhibits arachidonate 5-lipoxygenase (IC50 = 16 nM). |

STEARDA Dilution Calculator

STEARDA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.383 mL | 11.915 mL | 23.8299 mL | 47.6599 mL | 59.5749 mL |
| 5 mM | 0.4766 mL | 2.383 mL | 4.766 mL | 9.532 mL | 11.915 mL |
| 10 mM | 0.2383 mL | 1.1915 mL | 2.383 mL | 4.766 mL | 5.9575 mL |
| 50 mM | 0.0477 mL | 0.2383 mL | 0.4766 mL | 0.9532 mL | 1.1915 mL |
| 100 mM | 0.0238 mL | 0.1191 mL | 0.2383 mL | 0.4766 mL | 0.5957 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Doxycycline HCl
Catalog No.:BCC3772
CAS No.:10592-13-9
- Taraxasterol
Catalog No.:BCN5869
CAS No.:1059-14-9
- (tert-Butoxycarbonyl)oxycefcapene pivoxil
Catalog No.:BCC8403
CAS No.:105889-80-3
- Obtusilin
Catalog No.:BCN2697
CAS No.:105870-59-5
- TSTU
Catalog No.:BCC2828
CAS No.:105832-38-0
- Tropisetron Hydrochloride
Catalog No.:BCC4027
CAS No.:105826-92-4
- Nateglinide
Catalog No.:BCC5005
CAS No.:105816-04-4
- E-3810
Catalog No.:BCC1541
CAS No.:1058137-23-7
- Sitostenone
Catalog No.:BCN5868
CAS No.:1058-61-3
- Fmoc-Ser(tBu)-OPfp
Catalog No.:BCC3545
CAS No.:105751-13-1
- Methyl ganoderate C6
Catalog No.:BCN3259
CAS No.:105742-81-2
- Ganoderic acid C6
Catalog No.:BCN3257
CAS No.:105742-76-5
- OLDA
Catalog No.:BCC7138
CAS No.:105955-11-1
- Clinafloxacin CI96 AM1091
Catalog No.:BCC3754
CAS No.:105956-97-6
- Sulfocostunolide B
Catalog No.:BCN5870
CAS No.:1059671-65-6
- 2-(3,4-Dihydroxyphenyl)ethanol
Catalog No.:BCN5871
CAS No.:10597-60-1
- Geraniol
Catalog No.:BCN2631
CAS No.:106-24-1
- Nerol
Catalog No.:BCN8517
CAS No.:106-25-2
- β-Interleukin I (163-171), human
Catalog No.:BCC1017
CAS No.:106021-96-9
- Palmatine hydrochloride
Catalog No.:BCN5914
CAS No.:10605-02-4
- Hoechst 33342 analog 2
Catalog No.:BCC1631
CAS No.:106050-84-4
- Senktide
Catalog No.:BCC6921
CAS No.:106128-89-6
- PND-1186
Catalog No.:BCC1866
CAS No.:1061353-68-1
- TC-G 1004
Catalog No.:BCC6165
CAS No.:1061747-72-5
Modulation of inhibitory neurotransmission by the vanilloid receptor type 1 (TRPV1) in organotypically cultured mouse substantia gelatinosa neurons.[Pubmed:20451324]
Pain. 2010 Jul;150(1):128-40.
The vanilloid receptor type 1 (TRPV1) plays a pivotal role in modulating thermal, chemical, and inflammatory pain. TRPV1s are expressed in some dorsal horn (DH) neurons, but their contribution, if any, to central pain processing still remains unclear. We studied the effects of 2microM capsaicin-induced TRPV1 activation in organotypically cultured substantia gelatinosa neurons from post-natal (8-12) mice. Capsaicin affected sIPSC frequency (272+/-60% of control, n=14, P<0.02), but not amplitude (131+/-12% of control, n=14, P>0.05) in patch clamp recordings, also in the presence of 50microM AP-5 (frequency: 265+/-69% of control; n=8, P<0.05; amplitude: 156+/-28% of control; n=8, P>0.05). The frequency increase was reduced by TTX (181+/-21% of control; n=12, P<0.05). Pre-administration of I-RTX (1microM), a TRPV1 antagonist, prevented the capsaicin effect (frequency: 149+/-28% of control, P>0.05, n=12; amplitude: 97+/-4% of control, P>0.05, n=12). NADA (1microM), an endovanilloid/endocannabinoid agonist of TRPV1, induced a significant increase of sISPC frequency (191+/-40% of control; n=8, P<0.05) without affecting the amplitude (102+/-6% of control; n=8, P>0.05), and the co-application of two naturally occurring N-acyldopamines, PALDA (5microM) and STEARDA (5microM) that facilitate the effect of TRPV1 agonists, also induced a significant increase of sIPSC frequency (278+/-67% of control, n=6, P<0.05). The presence of TRPV1 protein and mRNA in DH neurons was confirmed by histological (immunocytochemistry, in situ PCR) and biochemical (Western blotting, PCR) procedures. These data show that TRPV1 modulates inhibitory neurotransmission in cultured substantia gelatinosa neurons, and suggest that endogenous agonists can activate the spinal receptors in vivo.
Actions of two naturally occurring saturated N-acyldopamines on transient receptor potential vanilloid 1 (TRPV1) channels.[Pubmed:15289293]
Br J Pharmacol. 2004 Sep;143(2):251-6.
Four long-chain, linear fatty acid dopamides (N-acyldopamines) have been identified in nervous bovine and rat tissues. Two unsaturated members of this family of lipids, N-arachidonoyl-dopamine (NADA) and N-oleoyl-dopamine, were shown to potently activate the transient receptor potential channel type V1 (TRPV1), also known as the vanilloid receptor type 1 for capsaicin. However, the other two congeners, N-palmitoyl- and N-stearoyl-dopamine (PALDA and STEARDA), are inactive on TRPV1. We have investigated here the possibility that the two compounds act by enhancing the effect of NADA on TRPV1 ('entourage' effect). When pre-incubated for 5 min with cells, both compounds dose-dependently enhanced NADA's TRPV1-mediated effect on intracellular Ca(2+) in human embryonic kidney cells overexpressing the human TRPV1. In the presence of either PALDA or STEARDA (0.1-10 microm), the EC(50) of NADA was lowered from approximately 90 to approximately 30 nm. The effect on intracellular Ca(2+) by another endovanilloid, N-arachidonoyl-ethanolamine (anandamide, 50 nm), was also enhanced dose-dependently by both PALDA and STEARDA. PALDA and STEARDA also acted in synergy with low pH (6.0-6.7) to enhance intracellular Ca(2+) via TRPV1. When co-injected with NADA (0.5 micrograms) in rat hind paws, STEARDA (5 micrograms) potentiated NADA's TRPV1-mediated nociceptive effect by significantly shortening the withdrawal latencies from a radiant heat source. STEARDA (1 and 10 micrograms) also enhanced the nocifensive behavior induced by carrageenan in a typical test of inflammatory pain. These data indicate that, despite their inactivity per se on TRPV1, PALDA and STEARDA may play a role as 'entourage' compounds on chemicophysical agents that interact with these receptors, with possible implications in inflammatory and neuropathic pain.
N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia.[Pubmed:12569099]
J Biol Chem. 2003 Apr 18;278(16):13633-9.
N-Arachidonoyldopamine (NADA) was recently identified as an endogenous ligand for the vanilloid type 1 receptor (VR1). Further analysis of the bovine striatal extract from which NADA was isolated indicated the existence of substances corresponding in molecular mass to N-oleoyldopamine (OLDA), N-palmitoyldopamine (PALDA), and N-stearoyldopamine (STEARDA). Quadrupole time-of-flight mass spectrometric analysis of bovine striatal extracts revealed the existence of OLDA, PALDA, and STEARDA as endogenous compounds in the mammalian brain. PALDA and STEARDA failed to affect calcium influx in VR1-transfected human embryonic kidney (HEK) 293 cells or paw withdrawal latencies from a radiant heat source, and there was no evidence of spontaneous pain behavior. By contrast, OLDA induced calcium influx (EC(50) = 36 nm), reduced the latency of paw withdrawal from a radiant heat source in a dose-dependent manner (EC(50) = 0.72 microg), and produced nocifensive behavior. These effects were blocked by co-administration of the VR1 antagonist iodo-resiniferatoxin (10 nm for HEK cells and 1 microg/50 micro;l for pain behavior). These findings demonstrate the existence of an endogenous compound in the brain that is similar to capsaicin and NADA in its chemical structure and activity on VR1. Unlike NADA, OLDA was only a weak ligand for rat CB1 receptors; but like NADA, it was recognized by the anandamide membrane transporter while being a poor substrate for fatty-acid amide hydrolase. Analysis of the activity of six additional synthetic and potentially endogenous N-acyldopamine indicated the requirement of a long unsaturated fatty acid chain for an optimal functional interaction with VR1 receptors.


