GeraniolCAS# 106-24-1 |
- Nerol
Catalog No.:BCN8517
CAS No.:106-25-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 106-24-1 | SDF | Download SDF |
| PubChem ID | 637566 | Appearance | Oil |
| Formula | C10H18O | M.Wt | 154.24 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
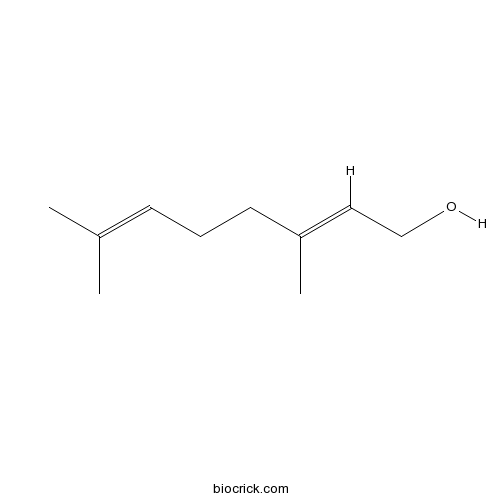
| Chemical Name | (2E)-3,7-dimethylocta-2,6-dien-1-ol | ||
| SMILES | CC(=CCCC(=CCO)C)C | ||
| Standard InChIKey | GLZPCOQZEFWAFX-JXMROGBWSA-N | ||
| Standard InChI | InChI=1S/C10H18O/c1-9(2)5-4-6-10(3)7-8-11/h5,7,11H,4,6,8H2,1-3H3/b10-7+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Geraniol is a terpene alcohol occurring in the essential oils of several aromatic plants used in the flavour and fragrance industries. It also exhibits insecticidal and repellent properties and used as a natural pest control agent exhibiting low toxicity.Geraniol is a glutathion S transferase inhibitor, shows anticarcinogenic props. |
| Targets | Calcium Channel | ATPase | Potassium Channel | Antifection |
| In vitro | Anti-Trichomonas vaginalis properties of the oil of Amomum tsao-ko and its major component, geraniol.[Pubmed: 25963227]Pharm Biol. 2015 May 12:1-6.Trichomonosis, caused by the flagellate protozoan Trichomonas vaginalis, is the most common non-viral sexually transmitted disease (STD) and 5-nitroimidazole drugs are used for the treatment. However, a growing number of T. vaginalis isolates are resistant to these drugs, which make it becomes an urgent issue.
The current study was designed to evaluate the anti-T. vaginalis activity of the essential oil from A. tsao-ko used in traditional Chinese medicine and as a spice and its main component, Geraniol.
Geraniol blocks calcium and potassium channels in the Mammalian myocardium: useful effects to treat arrhythmias.[Pubmed: 24862086]Basic Clin Pharmacol Toxicol. 2014 Dec;115(6):534-44.Geraniol is a monoterpene present in several essential oils, and it is known to have a plethora of pharmacological activities. In this study, we explored the contractile and electrophysiological properties of Geraniol and its antiarrhythmic effects in the heart. Investigating the antifungal activity and mechanism(s) of geraniol against Candida albicans strains.[Pubmed: 25480017]Med Mycol. 2015 Apr 1;53(3):275-84.Candida albicans can be a yeast that is a commensal on the human body but can cause opportunistic or pathogenic infections. Candida infections may create serious health problems and as a result has initiated a search for new drugs with an antifungal action. Geraniol is an acyclic monoterpene alcohol with known pharmacological properties, including antimicrobial activity. The aim of this work was to evaluate the antifungal activity and mechanism(s) of Geraniol against C. albicans strains. The minimum inhibitory concentration (MIC) was determined through broth microdilution techniques. |
| In vivo | Cross-reactivity between citral and geraniol - can it be attributed to oxidized geraniol?[Pubmed: 25209002]Contact Dermatitis. 2014 Nov;71(5):280-8.The fragrance compound Geraniol is susceptible to autoxidation when in contact with air, and to cutaneous metabolism. In both processes, the isomeric aldehydes geranial and neral are formed. Citral consists of geranial and neral. Among patients with positive reactions to citral, we have previously detected concomitant reactions to Geraniol in 85% of cases and to oxidized Geraniol in 73% of cases.
To study the pattern of concomitant reactions to Geraniol and citral and its isomers geranial and neral, and to determine whether these isomers are important sensitizers in contact allergy to Geraniol and oxidized Geraniol.
|

Geraniol Dilution Calculator

Geraniol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4834 mL | 32.417 mL | 64.834 mL | 129.668 mL | 162.0851 mL |
| 5 mM | 1.2967 mL | 6.4834 mL | 12.9668 mL | 25.9336 mL | 32.417 mL |
| 10 mM | 0.6483 mL | 3.2417 mL | 6.4834 mL | 12.9668 mL | 16.2085 mL |
| 50 mM | 0.1297 mL | 0.6483 mL | 1.2967 mL | 2.5934 mL | 3.2417 mL |
| 100 mM | 0.0648 mL | 0.3242 mL | 0.6483 mL | 1.2967 mL | 1.6209 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-(3,4-Dihydroxyphenyl)ethanol
Catalog No.:BCN5871
CAS No.:10597-60-1
- Sulfocostunolide B
Catalog No.:BCN5870
CAS No.:1059671-65-6
- Clinafloxacin CI96 AM1091
Catalog No.:BCC3754
CAS No.:105956-97-6
- OLDA
Catalog No.:BCC7138
CAS No.:105955-11-1
- STEARDA
Catalog No.:BCC7288
CAS No.:105955-10-0
- Doxycycline HCl
Catalog No.:BCC3772
CAS No.:10592-13-9
- Taraxasterol
Catalog No.:BCN5869
CAS No.:1059-14-9
- (tert-Butoxycarbonyl)oxycefcapene pivoxil
Catalog No.:BCC8403
CAS No.:105889-80-3
- Obtusilin
Catalog No.:BCN2697
CAS No.:105870-59-5
- TSTU
Catalog No.:BCC2828
CAS No.:105832-38-0
- Tropisetron Hydrochloride
Catalog No.:BCC4027
CAS No.:105826-92-4
- Nateglinide
Catalog No.:BCC5005
CAS No.:105816-04-4
- Nerol
Catalog No.:BCN8517
CAS No.:106-25-2
- β-Interleukin I (163-171), human
Catalog No.:BCC1017
CAS No.:106021-96-9
- Palmatine hydrochloride
Catalog No.:BCN5914
CAS No.:10605-02-4
- Hoechst 33342 analog 2
Catalog No.:BCC1631
CAS No.:106050-84-4
- Senktide
Catalog No.:BCC6921
CAS No.:106128-89-6
- PND-1186
Catalog No.:BCC1866
CAS No.:1061353-68-1
- TC-G 1004
Catalog No.:BCC6165
CAS No.:1061747-72-5
- WAY-600
Catalog No.:BCC4607
CAS No.:1062159-35-6
- WYE-687
Catalog No.:BCC4604
CAS No.:1062161-90-3
- WYE-354
Catalog No.:BCC1059
CAS No.:1062169-56-5
- Ro3280
Catalog No.:BCC3962
CAS No.:1062243-51-9
- LDN-193189
Catalog No.:BCC3687
CAS No.:1062368-24-4
Geraniol blocks calcium and potassium channels in the mammalian myocardium: useful effects to treat arrhythmias.[Pubmed:24862086]
Basic Clin Pharmacol Toxicol. 2014 Dec;115(6):534-44.
Geraniol is a monoterpene present in several essential oils, and it is known to have a plethora of pharmacological activities. In this study, we explored the contractile and electrophysiological properties of Geraniol and its antiarrhythmic effects in the heart. The Geraniol effects on atrial contractility, L-type Ca(2+) current, K(+) currents, action potential (AP) parameters, ECG profile and on the arrhythmia induced by ouabain were evaluated. In the atrium, Geraniol reduced the contractile force (~98%, EC = 1,510 +/- 160 muM) and diminished the positive inotropism of CaCl2 and BAY K8644. In cardiomyocytes, the IC a,L was reduced by 50.7% (n = 5) after perfusion with 300 muM Geraniol. Moreover, Geraniol prolonged the AP duration (APD) measured at 50% (n = 5) after repolarization, without changing the resting potential. The increased APD could be attributed to the blockade of the transient outward K(+) current (Ito ) (59.7%, n = 4), the non-inactivation K(+) current (Iss ) (39.2%, n = 4) and the inward rectifier K(+) current (IK 1 ) (33.7%, n = 4). In isolated hearts, Geraniol increased PRi and QTi without affecting the QRS complex (n = 6), and it reduced both the left ventricular pressure (83%) and heart rate (16.5%). Geraniol delayed the time to onset of ouabain-induced arrhythmias by 128%, preventing 30% of the increase in resting tension (n = 6). Geraniol exerts its negative inotropic and chronotropic responses in the heart by decreasing both L-type Ca(2+) and voltage-gated K(+) currents, ultimately acting against ouabain-induced arrhythmias.
Cross-reactivity between citral and geraniol - can it be attributed to oxidized geraniol?[Pubmed:25209002]
Contact Dermatitis. 2014 Nov;71(5):280-8.
BACKGROUND: The fragrance compound Geraniol is susceptible to autoxidation when in contact with air, and to cutaneous metabolism. In both processes, the isomeric aldehydes geranial and neral are formed. Citral consists of geranial and neral. Among patients with positive reactions to citral, we have previously detected concomitant reactions to Geraniol in 85% of cases and to oxidized Geraniol in 73% of cases. OBJECTIVE: To study the pattern of concomitant reactions to Geraniol and citral and its isomers geranial and neral, and to determine whether these isomers are important sensitizers in contact allergy to Geraniol and oxidized Geraniol. PATIENTS AND METHODS: The irritancy of geranial and citral was studied. Six hundred and fifty-five patients were patch tested with geranial, neral and citral at 3.5% pet., pure Geraniol at 6.0% and 11.0% pet., and oxidized Geraniol at 6.0% pet. RESULTS: Twenty-six per cent of citral-positive patients reacted to oxidized Geraniol, and 10.5% reacted to pure Geraniol. Citral and/or its isomers gave positive reactions in 25% of the patients who reacted to pure Geraniol. CONCLUSIONS: There is little cross-reactivity between pure Geraniol and citral; however, concomitant reactions to citral and oxidized Geraniol were common, owing to geranial. Geranial was also the main sensitizer in the mixture citral.
Investigating the antifungal activity and mechanism(s) of geraniol against Candida albicans strains.[Pubmed:25480017]
Med Mycol. 2015 Apr;53(3):275-84.
Candida albicans can be a yeast that is a commensal on the human body but can cause opportunistic or pathogenic infections. Candida infections may create serious health problems and as a result has initiated a search for new drugs with an antifungal action. Geraniol is an acyclic monoterpene alcohol with known pharmacological properties, including antimicrobial activity. The aim of this work was to evaluate the antifungal activity and mechanism(s) of Geraniol against C. albicans strains. The minimum inhibitory concentration (MIC) was determined through broth microdilution techniques. We investigated possible Geraniol activity on the fungal cell wall (sorbitol protect effect), cell membrane (Geraniol to ergosterol binding), the time-kill curve, and its biological activity on the yeast's morphology. Amphotericin B was used as control, and all tests were performed in duplicate. The MIC of Geraniol was 16 mug/ml (for 90% of isolates) but its probable mechanism of action did not involve the cell wall and ergosterol binding. In the morphological interference assay, we observed that the product inhibited pseudohyphae and chlamydoconidia formation. Time-dependent kill curve assay demonstrated that the fungicidal activity for MIC x 2 started at 2 h for the ATCC 76485 strain, and at 4 h for the LM-70 strain. Geraniol showed in vitro antifungal potential against strains of C. albicans but did not involve action on the cell wall or ergosterol. This study contributes to the development of new antifungal drugs, especially against Candida spp.
Anti-Trichomonas vaginalis properties of the oil of Amomum tsao-ko and its major component, geraniol.[Pubmed:25963227]
Pharm Biol. 2016;54(3):445-50.
CONTEXT: Trichomonosis, caused by the flagellate protozoan Trichomonas vaginalis, is the most common non-viral sexually transmitted disease (STD) and 5-nitroimidazole drugs are used for the treatment. However, a growing number of T. vaginalis isolates are resistant to these drugs, which make it becomes an urgent issue. OBJECTIVE: The current study was designed to evaluate the anti-T. vaginalis activity of the essential oil from A. tsao-ko used in traditional Chinese medicine and as a spice and its main component, Geraniol. MATERIALS AND METHODS: The anti-T. vaginalis activities of A. tsao-ko essential oil and Geraniol were evaluated by the minimum lethal concentration (MLC) and 50% inhibitory concentration (IC50) in vitro. The morphological changes of T. vaginalis were observed by transmission electron microscopy (TEM). Additionally, sub-MLC concentration treatment with sub-MLC A. tsao-ko essential oil and Geraniol was also performed. RESULTS: This study shows that MLC/IC50 of A. tsao-ko essential oil was 44.97 microg/ml/22.49 microg/ml for T. vaginalis isolate Tv1, and 89.93 microg/ml/44.97 microg/ml for T. vaginalis isolate Tv2. Those of Geraniol were 342.96 microg/ml/171.48 microg/ml, respectively. After A. tsao-ko essential oil or Geraniol treatment, obvious similar morphological changes of T. vaginalis were observed by TEM: the nuclear membrane was damaged, nuclei were dissolved, and the chromatin was accumulated; in the cytoplasm, numerous vacuoles appeared, rough endoplasmic reticulum dilated, the number of ribosomes were reduced, organelles disintegrated, the cell membrane was partially damaged, with cytoplasmic leakage, and cell disintegration was observed. The action time did not increase the effect of A. tsao-ko essential oil or Geraniol against T. vaginalis, as no significant difference was observed after sub-MLC concentration treatment for 1, 3, and 5 h with A. tsao-ko essential oil and Geraniol. DISCUSSION AND CONCLUSION: The study describes the first report on the activity and morphological changes of A. tsao-ko essential oil and Geraniol against T. vaginalis. The results obtained herein presented new opportunities for antitrichomonal drugs.


