LDN-193189ALK inhibitor,potent and selective CAS# 1062368-24-4 |
- ASP3026
Catalog No.:BCC1372
CAS No.:1097917-15-1
- CH5424802
Catalog No.:BCC3749
CAS No.:1256580-46-7
- SB 431542
Catalog No.:BCC3658
CAS No.:301836-41-9
- SB525334
Catalog No.:BCC2531
CAS No.:356559-20-1
- ALK inhibitor 1
Catalog No.:BCC1339
CAS No.:761436-81-1
- ALK inhibitor 2
Catalog No.:BCC1340
CAS No.:761438-38-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1062368-24-4 | SDF | Download SDF |
| PubChem ID | 25195294 | Appearance | Powder |
| Formula | C25H22N6 | M.Wt | 406.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DM-3189 | ||
| Solubility | Ethanol : 0.25 mg/mL (0.62 mM; Need ultrasonic and warming);H2O : < 0.1 mg/mL (insoluble);DMSO : < 1 mg/mL (insoluble or slightly soluble); | ||
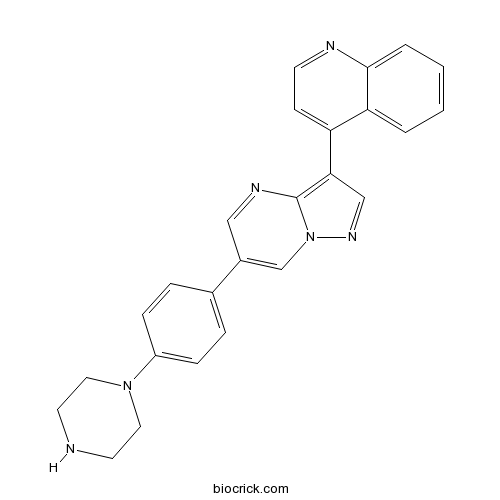
| Chemical Name | 4-[6-(4-piperazin-1-ylphenyl)pyrazolo[1,5-a]pyrimidin-3-yl]quinoline | ||
| SMILES | C1CN(CCN1)C2=CC=C(C=C2)C3=CN4C(=C(C=N4)C5=CC=NC6=CC=CC=C56)N=C3 | ||
| Standard InChIKey | CDOVNWNANFFLFJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H22N6/c1-2-4-24-22(3-1)21(9-10-27-24)23-16-29-31-17-19(15-28-25(23)31)18-5-7-20(8-6-18)30-13-11-26-12-14-30/h1-10,15-17,26H,11-14H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | LDN193189 is a selective inhibitor of BMP signaling for the BMP type I receptors ALK2 and ALK3 with IC50 of 5 nM and 30 nM, respectively. | |||||
| Targets | ALK2 | ALK3 | ||||
| IC50 | 5 nM | 30 nM | ||||

LDN-193189 Dilution Calculator

LDN-193189 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4601 mL | 12.3007 mL | 24.6015 mL | 49.2029 mL | 61.5036 mL |
| 5 mM | 0.492 mL | 2.4601 mL | 4.9203 mL | 9.8406 mL | 12.3007 mL |
| 10 mM | 0.246 mL | 1.2301 mL | 2.4601 mL | 4.9203 mL | 6.1504 mL |
| 50 mM | 0.0492 mL | 0.246 mL | 0.492 mL | 0.9841 mL | 1.2301 mL |
| 100 mM | 0.0246 mL | 0.123 mL | 0.246 mL | 0.492 mL | 0.615 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LDN193189 is a selective transcriptional activity morphogenetic protein (BMP) type I receptors inhibitor. It inhibits activin receptor-like kinase-2 (ALK2) and ALK3 with IC50 values of 5 nM and 30 nM, respectively [1].
LDN193189 has been showed to inhibit BMP induced phosphorylation of Smad signaling (Smad1/5/8) and non-Smad signaling including p38 and Akt in C2C12 cells [2].
Pharmacological inhibition of BMP by LDN193189 has shown to prevent down-regulation of E-cadherin in response to BMP2 both in bronchial epithelial (Beas2B) cells and in C57BL/6 mice. LDN193189 also inhibits the BMP-induced reduction of epithelial permeability at cellular level [3].
References:
[1] Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 2008 Dec;14(12):1363-9.
[2] Boergermann JH1, Kopf J, Yu PB, Knaus P. Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Int J Biochem Cell Biol. 2010 Nov;42(11):1802-7.
[3] Helbing T1, Herold EM, Hornstein A, Wintrich S, Heinke J, Grundmann S, Patterson C, Bode C, Moser M. Inhibition of BMP activity protects epithelial barrier function in lung injury. J Pathol. 2013 Sep;231(1):105-16. doi: 10.1002/path.4215. Epub 2013 Jul 10.
- Ro3280
Catalog No.:BCC3962
CAS No.:1062243-51-9
- WYE-354
Catalog No.:BCC1059
CAS No.:1062169-56-5
- WYE-687
Catalog No.:BCC4604
CAS No.:1062161-90-3
- WAY-600
Catalog No.:BCC4607
CAS No.:1062159-35-6
- TC-G 1004
Catalog No.:BCC6165
CAS No.:1061747-72-5
- PND-1186
Catalog No.:BCC1866
CAS No.:1061353-68-1
- Senktide
Catalog No.:BCC6921
CAS No.:106128-89-6
- Hoechst 33342 analog 2
Catalog No.:BCC1631
CAS No.:106050-84-4
- Palmatine hydrochloride
Catalog No.:BCN5914
CAS No.:10605-02-4
- β-Interleukin I (163-171), human
Catalog No.:BCC1017
CAS No.:106021-96-9
- Nerol
Catalog No.:BCN8517
CAS No.:106-25-2
- Geraniol
Catalog No.:BCN2631
CAS No.:106-24-1
- ML347
Catalog No.:BCC5331
CAS No.:1062368-49-3
- LDN193189 Hydrochloride
Catalog No.:BCC1695
CAS No.:1062368-62-0
- Thioperamide
Catalog No.:BCC6734
CAS No.:106243-16-7
- 4-[(4-Methylpiperazin-1-yl) methyl]benzoic acid dihydrochloride
Catalog No.:BCC8669
CAS No.:106261-49-8
- Risperidone
Catalog No.:BCC3850
CAS No.:106266-06-2
- Sikokianin A
Catalog No.:BCN3133
CAS No.:106293-99-6
- Nomilin
Catalog No.:BCN1034
CAS No.:1063-77-0
- Rufinamide
Catalog No.:BCC5078
CAS No.:106308-44-5
- DAPTA
Catalog No.:BCC5909
CAS No.:106362-34-9
- ω-Conotoxin GVIA
Catalog No.:BCC5700
CAS No.:106375-28-4
- Boc-D-Alaninol
Catalog No.:BCC2727
CAS No.:106391-86-0
- Boc-D-Valinol
Catalog No.:BCC2692
CAS No.:106391-87-1
Small molecules dorsomorphin and LDN-193189 inhibit myostatin/GDF8 signaling and promote functional myoblast differentiation.[Pubmed:25368322]
J Biol Chem. 2015 Feb 6;290(6):3390-404.
GDF8, or myostatin, is a member of the TGF-beta superfamily of secreted polypeptide growth factors. GDF8 is a potent negative regulator of myogenesis both in vivo and in vitro. We found that GDF8 signaling was inhibited by the small molecule ATP competitive inhibitors dorsomorphin and LDN-193189. These compounds were previously shown to be potent inhibitors of BMP signaling by binding to the BMP type I receptors ALK1/2/3/6. We present the crystal structure of the type II receptor ActRIIA with dorsomorphin and demonstrate that dorsomorphin or LDN-193189 target GDF8 induced Smad2/3 signaling and repression of myogenic transcription factors. As a result, both inhibitors rescued myogenesis in myoblasts treated with GDF8. As revealed by quantitative live cell microscopy, treatment with dorsomorphin or LDN-193189 promoted the contractile activity of myotubular networks in vitro. We therefore suggest these inhibitors as suitable tools to promote functional myogenesis.
Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells.[Pubmed:20691279]
Int J Biochem Cell Biol. 2010 Nov;42(11):1802-7.
Bone morphogenetic proteins (BMPs) are key regulators of cell fate decisions during embryogenesis and tissue homeostasis. BMPs signal through a coordinated assembly of two types of transmembrane serine/threonine kinase receptors to induce Smad1/5/8 plus non-Smad pathways, such as MAPK and Akt. The recent discovery of BMP receptor inhibitors opened new avenues to study specific BMP signalling and to delineate this effect from TGF-beta and Activin signalling. Here we present comprehensive and quantitative analyses on both canonical and non-Smad mediated BMP signalling under Dorsomorphin (DM) and LDN-193189 (LDN) treatment conditions. We demonstrate for the first time, that both compounds affect not only the Smad but also the non-Smad signalling pathways induced by either BMP2, BMP6 or GDF5. The activation of p38, ERK1/2 and Akt in C2C12 cells was inhibited by DM and LDN. In addition "off-target" effects on all branches of BMP non-Smad signalling are presented. From this we conclude that the inhibition of BMP receptors by DM and more efficiently by LDN-193189 affects all known BMP induced signalling cascades.
Differential cellular responses induced by dorsomorphin and LDN-193189 in chemotherapy-sensitive and chemotherapy-resistant human epithelial ovarian cancer cells.[Pubmed:25227893]
Int J Cancer. 2015 Mar 1;136(5):E455-69.
Inherent or acquired drug resistance is a major contributor to epithelial ovarian cancer (EOC) mortality. Novel drugs or drug combinations that produce EOC cell death or resensitize drug resistant cells to standard chemotherapy may improve patient treatment. After conducting drug tolerability studies for the multikinase inhibitors dorsomorphin (DM) and it is structural analogue LDN-193189 (LDN), these drugs were tested in a mouse intraperitoneal xenograft model of EOC. DM significantly increased survival, whereas LDN showed a trend toward increased survival. In vitro experiments using cisplatin (CP)-resistant EOC cell lines, A2780-cp or SKOV3, we determined that pretreatment or cotreatment with DM or LDN resensitized cells to the killing effect of CP or carboplatin (CB). DM was capable of blocking EOC cell cycle and migration, whereas LDN produced a less pronounced effect on cell cycle and no effect on migration. Subsequent analyses using primary human EOC cell samples or additional established EOC cells lines showed that DM or LDN induced a dose-dependent autophagic or cell death response, respectively. DM induced a characteristic morphological change with the appearance of numerous LC3B-containing acidic vacuoles and an increase in LC3BII levels. This was coincident with a decrease in cell growth and the altered cell cycle consistent with DM-induced cytostasis. By contrast, LDN produced a caspase 3-independent, reactive oxygen species-dependent cell death. Overall, DM and LDN possess drug characteristics suitable for adjuvant agents used to treat chemotherapy-sensitive and -resistant EOC.


