TC-G 1004Potent and selective A2A antagonist CAS# 1061747-72-5 |
- Nadifloxacin
Catalog No.:BCC4804
CAS No.:124858-35-1
- Calcipotriol monohydrate
Catalog No.:BCC1445
CAS No.:147657-22-5
- Halobetasol Propionate
Catalog No.:BCC4664
CAS No.:66852-54-8
- Dihydroartemisinin
Catalog No.:BCN6264
CAS No.:71939-50-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1061747-72-5 | SDF | Download SDF |
| PubChem ID | 25074316 | Appearance | Powder |
| Formula | C22H27N7O2 | M.Wt | 421.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. HCl and to 100 mM in DMSO | ||
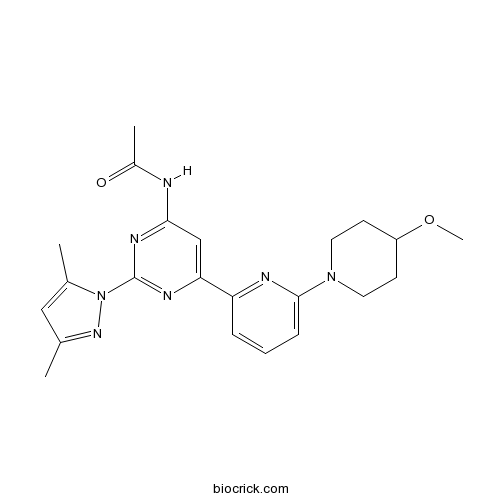
| Chemical Name | N-[2-(3,5-dimethylpyrazol-1-yl)-6-[6-(4-methoxypiperidin-1-yl)pyridin-2-yl]pyrimidin-4-yl]acetamide | ||
| SMILES | CC1=CC(=NN1C2=NC(=CC(=N2)NC(=O)C)C3=NC(=CC=C3)N4CCC(CC4)OC)C | ||
| Standard InChIKey | JENSDTKXNVHSSN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H27N7O2/c1-14-12-15(2)29(27-14)22-25-19(13-20(26-22)23-16(3)30)18-6-5-7-21(24-18)28-10-8-17(31-4)9-11-28/h5-7,12-13,17H,8-11H2,1-4H3,(H,23,25,26,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent antagonist of adenosine A2A receptors; displays >100-fold selectivity for A2A over A1 receptors (Ki values are 0.44 and 85 nM respectively). Potentiates L-DOPA-induced rotational behavior in 6-OHDA-lesioned rats. |

TC-G 1004 Dilution Calculator

TC-G 1004 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3725 mL | 11.8624 mL | 23.7248 mL | 47.4496 mL | 59.312 mL |
| 5 mM | 0.4745 mL | 2.3725 mL | 4.745 mL | 9.4899 mL | 11.8624 mL |
| 10 mM | 0.2372 mL | 1.1862 mL | 2.3725 mL | 4.745 mL | 5.9312 mL |
| 50 mM | 0.0474 mL | 0.2372 mL | 0.4745 mL | 0.949 mL | 1.1862 mL |
| 100 mM | 0.0237 mL | 0.1186 mL | 0.2372 mL | 0.4745 mL | 0.5931 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PND-1186
Catalog No.:BCC1866
CAS No.:1061353-68-1
- Senktide
Catalog No.:BCC6921
CAS No.:106128-89-6
- Hoechst 33342 analog 2
Catalog No.:BCC1631
CAS No.:106050-84-4
- Palmatine hydrochloride
Catalog No.:BCN5914
CAS No.:10605-02-4
- β-Interleukin I (163-171), human
Catalog No.:BCC1017
CAS No.:106021-96-9
- Nerol
Catalog No.:BCN8517
CAS No.:106-25-2
- Geraniol
Catalog No.:BCN2631
CAS No.:106-24-1
- 2-(3,4-Dihydroxyphenyl)ethanol
Catalog No.:BCN5871
CAS No.:10597-60-1
- Sulfocostunolide B
Catalog No.:BCN5870
CAS No.:1059671-65-6
- Clinafloxacin CI96 AM1091
Catalog No.:BCC3754
CAS No.:105956-97-6
- OLDA
Catalog No.:BCC7138
CAS No.:105955-11-1
- STEARDA
Catalog No.:BCC7288
CAS No.:105955-10-0
- WAY-600
Catalog No.:BCC4607
CAS No.:1062159-35-6
- WYE-687
Catalog No.:BCC4604
CAS No.:1062161-90-3
- WYE-354
Catalog No.:BCC1059
CAS No.:1062169-56-5
- Ro3280
Catalog No.:BCC3962
CAS No.:1062243-51-9
- LDN-193189
Catalog No.:BCC3687
CAS No.:1062368-24-4
- ML347
Catalog No.:BCC5331
CAS No.:1062368-49-3
- LDN193189 Hydrochloride
Catalog No.:BCC1695
CAS No.:1062368-62-0
- Thioperamide
Catalog No.:BCC6734
CAS No.:106243-16-7
- 4-[(4-Methylpiperazin-1-yl) methyl]benzoic acid dihydrochloride
Catalog No.:BCC8669
CAS No.:106261-49-8
- Risperidone
Catalog No.:BCC3850
CAS No.:106266-06-2
- Sikokianin A
Catalog No.:BCN3133
CAS No.:106293-99-6
- Nomilin
Catalog No.:BCN1034
CAS No.:1063-77-0
Lead optimization of 4-acetylamino-2-(3,5-dimethylpyrazol-1-yl)-6-pyridylpyrimidines as A2A adenosine receptor antagonists for the treatment of Parkinson's disease.[Pubmed:18947224]
J Med Chem. 2008 Nov 27;51(22):7099-110.
4-Acetylamino-2-(3,5-dimethylpyrazol-1-yl)-pyrimidines bearing substituted pyridyl groups as C-6 substituents were prepared as selective adenosine hA2A receptor antagonists for the treatment of Parkinson's disease. The 5-methoxy-3-pyridyl derivative 6g (hA2A Ki 2.3 nM, hA1 Ki 190 nM) was orally active at 3 mg/kg in a rat HIC model but exposure was poor in nonrodent species, presumably due to poor aqueous solubility. Follow-on compound 16a (hA2A Ki 0.83 nM, hA1 Ki 130 nM), bearing a 6-(morpholin-4-yl)-2-pyridyl substituent at C-6, had improved solubility and was orally efficacious (3 mg/kg, HIC) but showed time-dependent cytochrome P450 3A4 inhibition, possibly related to morpholine ring metabolism. Compound 16j (hA2A Ki 0.44 nM, hA1 Ki 80 nM), bearing a 6-(4-methoxypiperidin-1-yl)-2-pyridyl substituent at C-6, was sparingly soluble but had good oral exposure in rodent and nonrodent species, had no cytochrome P450 or human ether-a-go-go related gene channel issues, and was orally efficacious at 1 mg/kg in HIC and at 3 mg/kg for potentiation of l-dopa-induced contralateral rotations in 6-hydroxydopamine-lesioned rats.


