Halobetasol PropionateCAS# 66852-54-8 |
- Sabutoclax
Catalog No.:BCC2236
CAS No.:1228108-65-3
- ABT-199
Catalog No.:BCC3614
CAS No.:1257044-40-8
- BM-1074
Catalog No.:BCC2235
CAS No.:1391108-10-3
- ABT-737
Catalog No.:BCC3613
CAS No.:852808-04-9
- ABT-263 (Navitoclax)
Catalog No.:BCC1272
CAS No.:923564-51-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 66852-54-8 | SDF | Download SDF |
| PubChem ID | 48175 | Appearance | Powder |
| Formula | C25H31ClF2O5 | M.Wt | 484.96 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BMY-30056; CGP-14458; Ulobetasol propionate | ||
| Solubility | DMSO : ≥ 100 mg/mL (206.20 mM) *"≥" means soluble, but saturation unknown. | ||
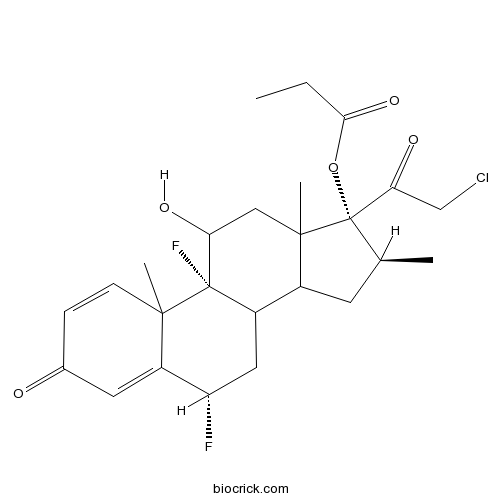
| Chemical Name | [(6S,9R,16S,17R)-17-(2-chloroacetyl)-6,9-difluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] propanoate | ||
| SMILES | CCC(=O)OC1(C(CC2C1(CC(C3(C2CC(C4=CC(=O)C=CC43C)F)F)O)C)C)C(=O)CCl | ||
| Standard InChIKey | BDSYKGHYMJNPAB-YKQIDFLYSA-N | ||
| Standard InChI | InChI=1S/C25H31ClF2O5/c1-5-21(32)33-25(20(31)12-26)13(2)8-15-16-10-18(27)17-9-14(29)6-7-22(17,3)24(16,28)19(30)11-23(15,25)4/h6-7,9,13,15-16,18-19,30H,5,8,10-12H2,1-4H3/t13-,15?,16?,18-,19?,22?,23?,24-,25-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Halobetasol propionate is a synthetic corticosteroid for topical dermatological use; exhibits anti-inflammatory, antipruritic, and vasoconstrictive properties. |

Halobetasol Propionate Dilution Calculator

Halobetasol Propionate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.062 mL | 10.3101 mL | 20.6203 mL | 41.2405 mL | 51.5506 mL |
| 5 mM | 0.4124 mL | 2.062 mL | 4.1241 mL | 8.2481 mL | 10.3101 mL |
| 10 mM | 0.2062 mL | 1.031 mL | 2.062 mL | 4.1241 mL | 5.1551 mL |
| 50 mM | 0.0412 mL | 0.2062 mL | 0.4124 mL | 0.8248 mL | 1.031 mL |
| 100 mM | 0.0206 mL | 0.1031 mL | 0.2062 mL | 0.4124 mL | 0.5155 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Halobetasol Propionate is an anti-inflammatory and a dermatologic agent commonly used to treat psoriasis.
- LDN 57444
Catalog No.:BCC2087
CAS No.:668467-91-2
- Linagliptin (BI-1356)
Catalog No.:BCC2110
CAS No.:668270-12-0
- Magnoflorine chloride
Catalog No.:BCN2405
CAS No.:6681-18-1
- Jatrorrhizine chloride
Catalog No.:BCN4956
CAS No.:6681-15-8
- Hernandezine
Catalog No.:BCN7793
CAS No.:6681-13-6
- (+/-)-Forbesione
Catalog No.:BCN6423
CAS No.:667914-50-3
- Syringaresinol-di-O-glucoside
Catalog No.:BCN2600
CAS No.:66791-77-3
- Impurity B of Calcitriol
Catalog No.:BCC1645
CAS No.:66791-71-7
- Platycodin A
Catalog No.:BCN7997
CAS No.:66779-34-8
- Esculin Sesquihydrate
Catalog No.:BCC8324
CAS No.:66778-17-4
- 6,8-Diprenylorobol
Catalog No.:BCN4602
CAS No.:66777-70-6
- Diosbulbin D
Catalog No.:BCN4218
CAS No.:66756-57-8
- H-D-Leu-OBzl.HCl
Catalog No.:BCC2682
CAS No.:66866-69-1
- Talniflumate
Catalog No.:BCC7391
CAS No.:66898-62-2
- 1,2-O-Isopropylidene-beta-D-fructopyranose
Catalog No.:BCN1383
CAS No.:66900-93-4
- α-Conotoxin PIA
Catalog No.:BCC5976
CAS No.:669050-68-4
- Beta-Belladonnine
Catalog No.:BCN1893
CAS No.:6696-63-5
- Tianeptine
Catalog No.:BCC1999
CAS No.:66981-73-5
- Thiamine hydrochloride
Catalog No.:BCN2225
CAS No.:67-03-8
- EGTA
Catalog No.:BCC7491
CAS No.:67-42-5
- Furazolidone
Catalog No.:BCC8988
CAS No.:67-45-8
- 5-Hydroxymethylfurfural
Catalog No.:BCN4226
CAS No.:67-47-0
- Fluocinolone Acetonide
Catalog No.:BCC4906
CAS No.:67-73-2
- Dicyclomine HCl
Catalog No.:BCC3762
CAS No.:67-92-5
Impurity profiling and a stability-indicating UPLC method development and validation for the estimation of related impurities of halobetasol propionate in halobetasol propionate 0.05% (w/w) cream.[Pubmed:24795078]
J Chromatogr Sci. 2015 Jan;53(1):112-21.
A simple, short and stability-indicating reverse phase-ultra-performance liquid chromatography method was developed and validated for the quantitative determination of related impurities of Halobetasol Propionate in Halobetasol Propionate 0.05% cream formulation. The proposed method was developed on an ACQUITY UPLC BEH Phenyl (2.1 x 100 mm, 1.7 microm) column at 40 degrees C with a mobile phase containing a gradient mixture of potassium hydrogen phosphate buffer and acetonitrile and methanol as modifiers with a runtime of 13.0 min at a monitored wavelength of 242 nm. A simple preparative method and liquid chromatography-mass spectrometry-compatible UPLC method also were developed for the isolation and identification of impurities and degradation products. The drug was subjected to forced-degradation conditions and found to degrade significantly. The stability-indicating capability of the developed method is established by analyzing forced-degradation samples in which the spectral purity of Halobetasol Propionate is ascertained along with the separation of degradation products from the analyte peak. The developed method was validated as per International Conference on Harmonization guidelines. The developed method is precise (%relative standard deviation <2.0) and is capable of detecting and quantifying all the six impurities at a level of 0.01 and 0.03%, respectively, with respect to test concentration. The wide linearity range, sensitivity, accuracy, short retention time and simple mobile phase imply that the method is suitable for routine quantification of Halobetasol Propionate and its related substances.
Two Multicenter, Randomized, Double-Blind, Parallel Group Comparison Studies of a Novel Enhanced Lotion Formulation of Halobetasol Propionate, 0.05% Versus Its Vehicle in Adult Subjects With Plaque Psoriasis.[Pubmed:28301619]
J Drugs Dermatol. 2017 Mar 1;16(3):234-240.
BACKGROUND: A novel lotion formulation of Halobetasol Propionate, 0.05% (HBP Lotion) with enhanced vehicle characteristics of a cream while preserving the ease of use and cosmetic elegance of a lotion has been developed to treat plaque psoriasis. OBJECTIVE: Determine the safety and effectiveness of HBP Lotion in patients with plaque psoriasis. METHODS: Two prospective, randomized, vehicle-controlled clinical studies were conducted in 443 adult subjects with moderate-severe plaque psoriasis. Subjects applied the test article to psoriatic plaques within the treatment area twice daily for 14 days. Efficacy data are based upon treatment "success" defined as those subjects that achieved scores of 0=clear or 1=almost clear with at least a two-grade improvement relative to baseline for an Investigator's Global Assessment (IGA) and clinical signs (plaque elevation, erythema, scaling). Safety data are presented as adverse events and local skin reactions. RESULTS: After two weeks of treatment with HBP Lotion, 44.5% of the HBP Lotion treated subjects in each study achieved (a) treatment "success" (ie, an IGA score of 0=clear or 1=almost clear and >2 grade improvement compared to baseline) and (b) a notable reduction in plaque elevation, erythema, scaling, and pruritus. In contrast, only 6.3% and 7.1% of VEH subjects in Studies 1 and 2, respectively, achieved treatment success and the reduction of disease related signs was materially lower. Statistically, at day 15 in both Phase 3 studies, treatment success with HBP Lotion was superior to VEH (P less than 0.001). From a safety perspective the outcomes were in general unremarkable with similar findings in the HBP Lotion and VEH treatment groups. CONCLUSIONS: The results demonstrate the safety and effectiveness of HBP Lotion in the treatment of plaque psoriasis. Furthermore, this novel HBP lotion formulation is also distinguished by its moisturization qualities and ease of use.
J Drugs Dermatol. 2017;16(3):234-240.
.Halobetasol Propionate Lotion, 0.05% Provides Superior Hydration Compared to Halobetasol Propionate Cream, 0.05% in a Double-Blinded Study of Occlusivity and Hydration.[Pubmed:28300856]
J Drugs Dermatol. 2017 Feb 1;16(2):140-144.
BACKGROUND: This study measured skin hydration and occlusivity of two test products [Halobetasol Propionate lotion, 0.05% (HBP Lotion) and Ultravate(R) (Halobetasol Propionate) cream, 0.05% (HBP Cream)] at 2, 4, and 6 hours after application to skin test sites previously challenged by dry shaving, which was performed to compromise the integrity of the stratum corneum barrier. METHODS: Trans-epidermal water loss (TEWL), an indicator of skin barrier function, was measured using cyberDERM, inc. RG-1 evaporimeter. Skin hydration was evaluated using IBS SkiCon-200 conductance meter. Test products were applied bilaterally on dry-shaved sites on the volar forearm sites, according to a randomization scheme, with two test sites untreated to serve as "dry-shaved" controls. TEWL and conductance were measured at 2, 4, and 6 hours post-treatment. RESULTS: HBP Lotion displayed a significant increase in skin hydration at 2, 4, and 6 hours post-treatment compared to the baseline values and dry-shaved controls (each, P less than 0.001). However, HBP Cream produced statistically significant increased skin hydration only after 6 hours (P less than 0.05). HBP Lotion was significantly more effective than HBP Cream in increasing skin hydration at 2 and 4 hours post-treatment (each, P less than 0.001), and had a directional advantage (not statistically significant) at 6 hours. Neither test product had a significant occlusive effect as measured by TEWL at 2, 4, and 6 hours post-application. CONCLUSION: Both formulations of HBP (Lotion and Cream) contributed to skin moisturization, as measured by skin conductance. HBP Lotion produced a significantly more rapid onset and higher level of moisturization at 2 and 4 hours post-application compared to HBP Cream. The TEWL results indicate that neither HBP Lotion nor HBP Cream provided any significant occlusivity to the skin.
J Drugs Dermatol. 2017;16(2):140-144.
.Quantification of halobetasol propionate and its impurities present in topical dosage forms by stability-indicating LC method.[Pubmed:24784115]
J Chromatogr Sci. 2015 Jan;53(1):127-34.
A novel, sensitive, stability-indicating, gradient, reverse-phase high-performance liquid chromatographic method has been developed for quantitative determination of Halobetasol Propionate and its impurities in topical dosage forms. The chromatographic separation was achieved on a Phenomenex Synergi polar reverse phase, 250 x 4.6 mm, 4 microm column. Mobile phase A comprises a mixture of 0.01 M KH2PO4 buffer containing 0.2% 1-octane sulfonic acid sodium salt (pH 3.0), acetonitrile and methanol in the ratio 80:15:05 (v/v/v), respectively, and mobile phase B contains a mixture of 0.01 M KH2PO4 buffer containing 0.2% 1-octane sulfonic acid sodium salt (pH 3.0), acetonitrile and methanol in the ratio 20:70:10 (v/v/v), respectively. The flow rate is 0.8 mL min(-1). The column compartment temperature is set at 40 degrees C and the detection wavelength is set at 240 nm. The resolutions between Halobetasol Propionate and all the impurities are >2.0 for all pairs of compounds. The drug product was subjected to International Conference on Harmonization (ICH)-prescribed hydrolytic, oxidative, photolytic and thermal stress conditions. The method is validated as per the ICH guidelines with respect to specificity, linearity, limit of detection, limit of quantification, accuracy, precision, robustness and ruggedness.


