LDN 57444UCH-L1 inhibitor,reversible competitve CAS# 668467-91-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 668467-91-2 | SDF | Download SDF |
| PubChem ID | 24906255 | Appearance | Powder |
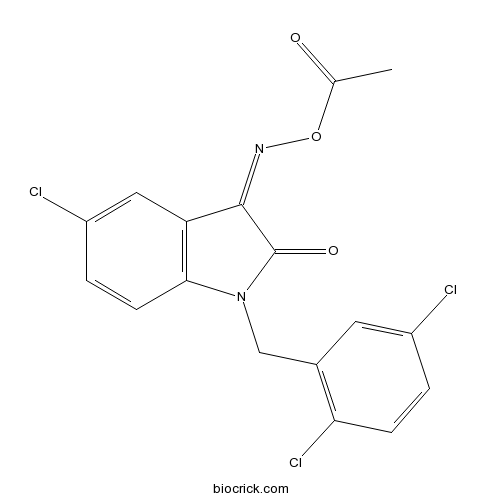
| Formula | C17H11Cl3N2O3 | M.Wt | 397.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 25 mg/mL (62.87 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | [[5-chloro-1-[(2,5-dichlorophenyl)methyl]-2-oxoindol-3-ylidene]amino] acetate | ||
| SMILES | CC(=O)ON=C1C2=C(C=CC(=C2)Cl)N(C1=O)CC3=C(C=CC(=C3)Cl)Cl | ||
| Standard InChIKey | OPQRFPHLZZPCCH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H11Cl3N2O3/c1-9(23)25-21-16-13-7-12(19)3-5-15(13)22(17(16)24)8-10-6-11(18)2-4-14(10)20/h2-7H,8H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of ubiquitin C-terminal hydrolase-L1 (UCH-L1) activity (Ki = 0.4 μM). Causes cell death through the apoptosis pathway; increases levels of highly ubiquitinated proteins and decreases ubiquitin proteasome activity. Activity leads to dramatic alterations in synaptic protein distribution and spine morphology in vivo. |

LDN 57444 Dilution Calculator

LDN 57444 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5148 mL | 12.5742 mL | 25.1484 mL | 50.2968 mL | 62.8709 mL |
| 5 mM | 0.503 mL | 2.5148 mL | 5.0297 mL | 10.0594 mL | 12.5742 mL |
| 10 mM | 0.2515 mL | 1.2574 mL | 2.5148 mL | 5.0297 mL | 6.2871 mL |
| 50 mM | 0.0503 mL | 0.2515 mL | 0.503 mL | 1.0059 mL | 1.2574 mL |
| 100 mM | 0.0251 mL | 0.1257 mL | 0.2515 mL | 0.503 mL | 0.6287 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LDN 57444 is a reversible competitive inhibitor of ubiquitin C-terminal hydrolase L1 (Uch-L1) with IC50 value of 0.88μM [1].
Besides Uch-L1, LDN 57444 also inhibits Uch-L3 with a higher IC50 value of 25μM. The inhibition of Uch enzymes subsequently causes the reduction of long-term potentiation and basal synaptic transmission, which is similar to the alterations induced by Aβ [1].
In rat insulinoma cell line INS 832/13, treatment of LDN 57444 leads to the cell apoptosis. The increased activity of caspase-3 can be seen when the concentration of LDN 57444 is above 30μM. LDN 57444 also induces nuclear CHOP, suggesting that the apoptosis induced by the inhibition of Uch-L1 is related to ER stress [2].
References:
[1] Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006 Aug 25;126(4):775-88.
[2] Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006 Aug 25;126(4):775-88.
- Linagliptin (BI-1356)
Catalog No.:BCC2110
CAS No.:668270-12-0
- Magnoflorine chloride
Catalog No.:BCN2405
CAS No.:6681-18-1
- Jatrorrhizine chloride
Catalog No.:BCN4956
CAS No.:6681-15-8
- Hernandezine
Catalog No.:BCN7793
CAS No.:6681-13-6
- (+/-)-Forbesione
Catalog No.:BCN6423
CAS No.:667914-50-3
- Syringaresinol-di-O-glucoside
Catalog No.:BCN2600
CAS No.:66791-77-3
- Impurity B of Calcitriol
Catalog No.:BCC1645
CAS No.:66791-71-7
- Platycodin A
Catalog No.:BCN7997
CAS No.:66779-34-8
- Esculin Sesquihydrate
Catalog No.:BCC8324
CAS No.:66778-17-4
- 6,8-Diprenylorobol
Catalog No.:BCN4602
CAS No.:66777-70-6
- Diosbulbin D
Catalog No.:BCN4218
CAS No.:66756-57-8
- NSC 109555 ditosylate
Catalog No.:BCC7540
CAS No.:66748-43-4
- Halobetasol Propionate
Catalog No.:BCC4664
CAS No.:66852-54-8
- H-D-Leu-OBzl.HCl
Catalog No.:BCC2682
CAS No.:66866-69-1
- Talniflumate
Catalog No.:BCC7391
CAS No.:66898-62-2
- 1,2-O-Isopropylidene-beta-D-fructopyranose
Catalog No.:BCN1383
CAS No.:66900-93-4
- α-Conotoxin PIA
Catalog No.:BCC5976
CAS No.:669050-68-4
- Beta-Belladonnine
Catalog No.:BCN1893
CAS No.:6696-63-5
- Tianeptine
Catalog No.:BCC1999
CAS No.:66981-73-5
- Thiamine hydrochloride
Catalog No.:BCN2225
CAS No.:67-03-8
- EGTA
Catalog No.:BCC7491
CAS No.:67-42-5
- Furazolidone
Catalog No.:BCC8988
CAS No.:67-45-8
- 5-Hydroxymethylfurfural
Catalog No.:BCN4226
CAS No.:67-47-0
- Fluocinolone Acetonide
Catalog No.:BCC4906
CAS No.:67-73-2
Functional modulation on macrophage by low dose naltrexone (LDN).[Pubmed:27561742]
Int Immunopharmacol. 2016 Oct;39:397-402.
Previously it was confirmed that naltrexone, a non-peptide delta-opioid receptor selective antagonist is mainly used for alcoholic dependence and opioid addiction treatment. However, there is increasing data on immune regulation of low dose naltrexone (LDN). The aim of this work was to explore the effect of LDN on the phenotype and function of macrophage. The changes of macrophage after treatment with LDN were examined using flow cytometry (FCM); FITC-dextran phagocytosis and enzyme-linked immunosorbent assay (ELISA). We have found that LDN enhances function of macrophage as confirmed by up-regulating MHC II molecule and CD64 on macrophage while down-regulating CD206 expression. Furthermore the productions of TNF-alpha, IL-6, IL-1beta, increased significantly. Macrophages in LDN treated group performed the enhanced phagocytosis. Therefore it is concluded that LDN could promote function of macrophage and this work has provided concrete data of impact on immune system by LDN. Especially the data would support interaction between CD4+T cell and macrophage in AIDS treatment with LDN in Africa (LDN has already been approved in Nigeria for the use in AIDS treatment).
A sudden and unprecedented increase in low dose naltrexone (LDN) prescribing in Norway. Patient and prescriber characteristics, and dispense patterns. A drug utilization cohort study.[Pubmed:27670755]
Pharmacoepidemiol Drug Saf. 2017 Feb;26(2):136-142.
PURPOSE: Following a TV documentary in 2013, there was a tremendous increase in low dose naltrexone (LDN) use in a wide range of unapproved indications in Norway. We aim to describe the extent of this sudden and unprecedented increase in LDN prescribing, to characterize patients and LDN prescribers, and to estimate LDN dose sizes. METHODS: LDN prescriptions recorded in the Norwegian Prescription Database (NorPD) in 2013 and 2014, and sales data not recorded in NorPD from the only Norwegian LDN manufacturer were included in the study. RESULTS: According to NorPD, 15 297 patients (0.3% of population) collected at least one LDN prescription. The actual number of users was higher as at least 23% of total sales were not recorded in NorPD. After an initial wave, there was a steady stream of new and persistent users throughout the study period. Median patient age was 52 years, and 74% of patients were female. Median daily dose was 3.7 mg. Twenty percent of all doctors and 71% of general medicine practitioners registered in Norway in 2014 prescribed LDN at least once. CONCLUSIONS: The TV documentary on LDN in Norway was followed by a large increase in LDN prescribing, and the proportion of LDN users went from an insignificant number to 0.3% of the population. There was a high willingness to use and prescribe off label despite limited evidence. Observed median LDN dose, and age and gender distribution were as expected in typical LDN using patients. (c) 2016 The Authors. Pharmacoepidemiology and Drug Safety Published by John Wiley & Sons Ltd.
Regulation of synaptic structure by ubiquitin C-terminal hydrolase L1.[Pubmed:19535597]
J Neurosci. 2009 Jun 17;29(24):7857-68.
Ubiquitin C-terminal hydrolase L1 (UCH-L1) is a deubiquitinating enzyme that is selectively and abundantly expressed in the brain, and its activity is required for normal synaptic function. Here, we show that UCH-L1 functions in maintaining normal synaptic structure in hippocampal neurons. We found that UCH-L1 activity is rapidly upregulated by NMDA receptor activation, which leads to an increase in the levels of free monomeric ubiquitin. Conversely, pharmacological inhibition of UCH-L1 significantly reduces monomeric ubiquitin levels and causes dramatic alterations in synaptic protein distribution and spine morphology. Inhibition of UCH-L1 activity increases spine size while decreasing spine density. Furthermore, there is a concomitant increase in the size of presynaptic and postsynaptic protein clusters. Interestingly, however, ectopic expression of ubiquitin restores normal synaptic structure in UCH-L1-inhibited neurons. These findings point to a significant role of UCH-L1 in synaptic remodeling, most likely by modulating free monomeric ubiquitin levels in an activity-dependent manner.
Endoplasmic reticulum stress contributes to the cell death induced by UCH-L1 inhibitor.[Pubmed:18622688]
Mol Cell Biochem. 2008 Nov;318(1-2):109-15.
At the neuropathological level, Parkinson's disease (PD) is characterized by the accumulation of misfolded proteins, which can trigger the unfolded protein response (UPR). UCH-L1 is a component of ubiquitin proteasome system (UPS). It is reported that the loss of its function will impair ubiquitin proteasome system and cause toxicity to cells. But its mechanism has not been illustrated. In this study, we detected the protein expression of Bip/Grp78 and the spliced form of XBP-1 to examine the activation of unfolded protein response after SK-N-SH cells being treated with LDN-57444, a UCH-L1 inhibitor which could inhibit UCH-L1 hydrolase activity. Our data showed that UCH-L1 inhibitor was able to cause cell death through the apoptosis pathway by decreasing the activity of ubiquitin proteasome system and increasing the levels of highly ubiquitinated proteins, both of which can activate unfolded protein response. There is a lot of evidence that unfolded protein response is activated as a protective response at the early stage of the stress; this protective response can switch to a pro-apoptotic response when the stress persists. In this study, we demonstrated this switch by detecting the upregulation of CHOP/Gadd153. Taken together, our data indicated that the apoptosis induced by UCH-L1 inhibitor may be triggered by the activation of endoplasmic reticulum stress (ERS). Moreover, we provide a new cell model for studying the roles of UCH-L1 in Parkinson's disease.
Discovery of inhibitors that elucidate the role of UCH-L1 activity in the H1299 lung cancer cell line.[Pubmed:14522054]
Chem Biol. 2003 Sep;10(9):837-46.
Neuronal ubiquitin C-terminal hydrolase (UCH-L1) has been linked to Parkinson's disease (PD), the progression of certain nonneuronal tumors, and neuropathic pain. Certain lung tumor-derived cell lines express UCH-L1 but it is not expressed in normal lung tissue, suggesting that this enzyme plays a role in tumor progression, either as a trigger or as a response. Small-molecule inhibitors of UCH-L1 would be helpful in distinguishing between these scenarios. By utilizing high-throughput screening (HTS) to find inhibitors and traditional medicinal chemistry to optimize their affinity and specificity, we have identified a class of isatin O-acyl oximes that selectively inhibit UCH-L1 as compared to its systemic isoform, UCH-L3. Three representatives of this class (30, 50, 51) have IC(50) values of 0.80-0.94 micro M for UCH-L1 and 17-25 micro M for UCH-L3. The K(i) of 30 toward UCH-L1 is 0.40 micro M and inhibition is reversible, competitive, and active site directed. Two isatin oxime inhibitors increased proliferation of the H1299 lung tumor cell line but had no effect on a lung tumor line that does not express UCH-L1. Inhibition of UCH-L1 expression in the H1299 cell line using RNAi had a similar proproliferative effect, suggesting that the UCH-L1 enzymatic activity is antiproliferative and that UCH-L1 expression may be a response to tumor growth. The molecular mechanism of this response remains to be determined.


