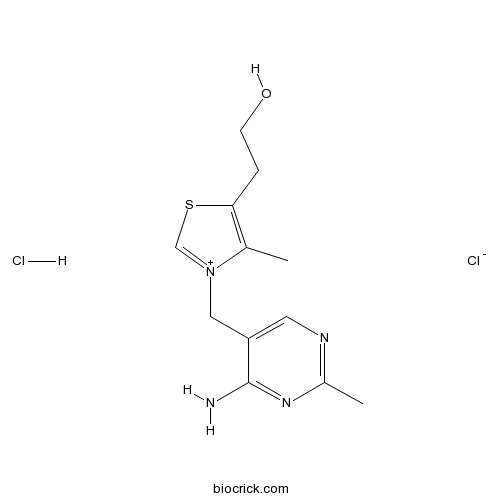
Thiamine hydrochlorideCAS# 67-03-8 |
- Lenalidomide hydrochloride
Catalog No.:BCC1697
CAS No.:1243329-97-6
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- Necrostatin 2 racemate
Catalog No.:BCC2077
CAS No.:852391-15-2
- Necrostatin 2
Catalog No.:BCC1793
CAS No.:852391-19-6
- Necrostatin 2 S enantiomer
Catalog No.:BCC2078
CAS No.:852391-20-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 67-03-8 | SDF | Download SDF |
| PubChem ID | 6202 | Appearance | White cryst. |
| Formula | C12H18Cl2N4OS | M.Wt | 337.27 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | DMSO : 10 mg/mL (29.65 mM; Need ultrasonic) | ||
| Chemical Name | 2-[3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-4-methyl-1,3-thiazol-3-ium-5-yl]ethanol;chloride;hydrochloride | ||
| SMILES | CC1=C(SC=[N+]1CC2=CN=C(N=C2N)C)CCO.Cl.[Cl-] | ||
| Standard InChIKey | DPJRMOMPQZCRJU-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C12H17N4OS.2ClH/c1-8-11(3-4-17)18-7-16(8)6-10-5-14-9(2)15-12(10)13;;/h5,7,17H,3-4,6H2,1-2H3,(H2,13,14,15);2*1H/q+1;;/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Thiamine hydrochloride is an efficient catalyst for the synthesis of amidoalkyl naphthols, it has prophylactic potential on lead induced lipid peroxidation in rat liver and kidney. Thiamine hydrochloride complex as a new anti-diabetic candidate. |
| Targets | Dopamine Receptor |
| In vitro | Effect of thiamine hydrochloride, pyridoxine hydrochloride and calcium-d-pantothenate on the patulin content of apple juice concentrate.[Pubmed: 12224421]Nahrung. 2002 Aug;46(4):256-7.Thiamine hydrochloride, pyridoxine hydrochloride and calcium-d-pantothenate were applied apple juice concentrates (AJC) at various doses in order to reduce the patulin content. |
| In vivo | Pharmacokinetic study of benfotiamine and the bioavailability assessment compared to thiamine hydrochloride.[Pubmed: 24399744]J Clin Pharmacol. 2014 Jun;54(6):688-95.Benfotiamine is a lipid-soluble thiamine precursor which can transform to thiamine in vivo and subsequently be metabolized to thiamine monophosphate (TMP) and thiamine diphosphate (TDP). Synthesis, characterization, and efficacy evaluation of a new anti-diabetic vanadyl(II) thiamine hydrochloride complex in streptozotocin-induced diabetic rats.[Pubmed: 25816395]Int J Immunopathol Pharmacol. 2015 Mar 26.Diabetes mellitus (DM) is a chronic metabolic disorder characterized by hyperglycemia due to abnormalities in either insulin secretion or action. A range of vanadium complexes have been synthesized and demonstrated to be effective in lowering hyperglycemia. Thiamine administration was also reported to prevent deterioration in fasting glucose and insulin levels, and to improve glucose tolerance in hyperglycemic patients. |
| Animal Research | Effect of thiamine hydrochloride on lead induced lipid peroxidation in rat liver and kidney.[Pubmed: 10928693]Vet Hum Toxicol. 2000 Aug;42(4):236-7.
|
| Structure Identification | Tetrahedron Letters, 2009, 50(46):6393-6397.Thiamine Hydrochloride as an Efficient Catalyst for the Synthesis of Amidoalkyl Naphthols.[Reference: WebLink]A simple, efficient, and practical procedure for the synthesis of amidoalkyl naphthols using Thiamine hydrochloride (VB1) as a novel catalyst is described in high yields. The salient features of the catalyst are efficiency, inexpensiveness, non-toxicity, and metal ion free. |

Thiamine hydrochloride Dilution Calculator

Thiamine hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.965 mL | 14.8249 mL | 29.6498 mL | 59.2997 mL | 74.1246 mL |
| 5 mM | 0.593 mL | 2.965 mL | 5.93 mL | 11.8599 mL | 14.8249 mL |
| 10 mM | 0.2965 mL | 1.4825 mL | 2.965 mL | 5.93 mL | 7.4125 mL |
| 50 mM | 0.0593 mL | 0.2965 mL | 0.593 mL | 1.186 mL | 1.4825 mL |
| 100 mM | 0.0296 mL | 0.1482 mL | 0.2965 mL | 0.593 mL | 0.7412 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Thiamine or vitamin B1 is a water-soluble vitamin of the B complex, its phosphate derivatives are involved in many cellular processes.
- Tianeptine
Catalog No.:BCC1999
CAS No.:66981-73-5
- Beta-Belladonnine
Catalog No.:BCN1893
CAS No.:6696-63-5
- α-Conotoxin PIA
Catalog No.:BCC5976
CAS No.:669050-68-4
- 1,2-O-Isopropylidene-beta-D-fructopyranose
Catalog No.:BCN1383
CAS No.:66900-93-4
- Talniflumate
Catalog No.:BCC7391
CAS No.:66898-62-2
- H-D-Leu-OBzl.HCl
Catalog No.:BCC2682
CAS No.:66866-69-1
- Halobetasol Propionate
Catalog No.:BCC4664
CAS No.:66852-54-8
- LDN 57444
Catalog No.:BCC2087
CAS No.:668467-91-2
- Linagliptin (BI-1356)
Catalog No.:BCC2110
CAS No.:668270-12-0
- Magnoflorine chloride
Catalog No.:BCN2405
CAS No.:6681-18-1
- Jatrorrhizine chloride
Catalog No.:BCN4956
CAS No.:6681-15-8
- Hernandezine
Catalog No.:BCN7793
CAS No.:6681-13-6
- EGTA
Catalog No.:BCC7491
CAS No.:67-42-5
- Furazolidone
Catalog No.:BCC8988
CAS No.:67-45-8
- 5-Hydroxymethylfurfural
Catalog No.:BCN4226
CAS No.:67-47-0
- Fluocinolone Acetonide
Catalog No.:BCC4906
CAS No.:67-73-2
- Dicyclomine HCl
Catalog No.:BCC3762
CAS No.:67-92-5
- Vitamin D3
Catalog No.:BCN2186
CAS No.:67-97-0
- Gliotoxin
Catalog No.:BCN3894
CAS No.:67-99-2
- Anthriscusin
Catalog No.:BCN3533
CAS No.:67008-16-6
- Methyl isovanillate
Catalog No.:BCN7960
CAS No.:6702-50-7
- 3,4-Dihydroxy-2-methoxyxanthone
Catalog No.:BCN7631
CAS No.:6702-55-2
- Crenolanib (CP-868596)
Catalog No.:BCC3671
CAS No.:670220-88-9
- Ohchinin
Catalog No.:BCN4601
CAS No.:67023-80-7
Effect of thiamine hydrochloride, pyridoxine hydrochloride and calcium-d-pantothenate on the patulin content of apple juice concentrate.[Pubmed:12224421]
Nahrung. 2002 Aug;46(4):256-7.
Thiamine hydrochloride, pyridoxine hydrochloride and calcium-d-pantothenate were applied apple juice concentrates (AJC) at various doses in order to reduce the patulin content. AJC samples containing high levels of patulin were stored at 22 +/- 2 degrees C and 4 degrees C for 6 months after vitamins were added. Patulin was fully degraded at the end of a 6-month period in samples stored at 22 +/- 2 degrees C, on the other hand, other quality parameters diminished significantly. Without any considerable reduction on other quality parameters, applications of 1000 and 2500 mg/kg calcium-d-pantothenate resulted in reduction of patulin of 73.6 and 94.3%, respectively, however, 42.1% of patulin reduction was observed in the control sample of AJC stored for 1 month at 22 +/- 2 degrees C. Addition of Thiamine hydrochloride (1000 mg/kg), pyrodoxine hydrochloride (625 or 875 mg/kg) and calcium-d-pantothenate (1000 or 2500 mg/kg) into the samples and storage at 4 degrees C for 6 months yielded 55.5 to 67.7% of patulin reduction which was only 35.8% for the control while the other quality parameters were protected adequately.
Effect of thiamine hydrochloride on lead induced lipid peroxidation in rat liver and kidney.[Pubmed:10928693]
Vet Hum Toxicol. 2000 Aug;42(4):236-7.
Thiamine hydrochloride was studied on lead-induced endogenous lipid peroxidation in rat hepatic and renal tissues following po doses of 2.73 mg lead/kg bw for 6 w. Simultaneous use of 25 mg Thiamine hydrochloride/kg bw po reduced lead accumulation in liver and kidneys. There were significant decreases in endogenous lipid peroxide in liver and kidney from Thiamine hydrochloride-treated rats. Histopathological lesions in thiamine-treated livers and kidneys were milder in comparison to lesions in untreated Pb-exposed animals. This indicates the prophylactic potential of thiamine for lead-induced lipid peroxidation.
Synthesis, characterization, and efficacy evaluation of a new anti-diabetic vanadyl(II) thiamine hydrochloride complex in streptozotocin-induced diabetic rats.[Pubmed:25816395]
Int J Immunopathol Pharmacol. 2015 Jun;28(2):227-39.
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by hyperglycemia due to abnormalities in either insulin secretion or action. A range of vanadium complexes have been synthesized and demonstrated to be effective in lowering hyperglycemia. Thiamine administration was also reported to prevent deterioration in fasting glucose and insulin levels, and to improve glucose tolerance in hyperglycemic patients. This study has been conducted to evaluate the ionic vanadyl(II) Thiamine hydrochloride complex (VC) as a new anti-diabetic candidate. The new complex was characterized by infrared spectroscopy (FT-IR), electronic spectra, magnetic susceptibility, electron spin resonance (ESR), scanning electron microscopy (SEM), and thermogravimetric analysis (TGA). The anti-diabetic effect of VC was investigated in comparison to vanadium sulfate in streptozotocin (STZ)-induced diabetic rats. Treatment of diabetic rats with VC versus vanadyl sulfate showed a more potent effect on reducing serum glucose and cholesterol close to normal levels. VC suppressed the diabetes-induced upregulation of hepatic glucose transporter (GLUT)-2, Phosphoenol pyruvate carboxykinase (PEPCK), and hormone-sensitive lipase (HSL) more significantly than vanadyl sulfate. Either vanadyl sulfate or VC restored hepatic sterol regulatory element-binding protein transcription factor-1c (SREBP-1c) and muscle hexokinase (HK) mRNA expression that was downregulated in diabetic group. Pyruvate kinase (PK) mRNA expression was restored more significantly in VC-treated than vanadyl sulfate-treated diabetic rats. These results indicate that the newly synthesized VC could be an effective anti-diabetic candidate as the anti-diabetic activity of the ionic vanadium was enhanced after being modified with the organic ligand, thiamin. The results also suggest that VC achieves its effect most likely through modulating the transcription of energy metabolizing enzymes.
Pharmacokinetic study of benfotiamine and the bioavailability assessment compared to thiamine hydrochloride.[Pubmed:24399744]
J Clin Pharmacol. 2014 Jun;54(6):688-95.
Benfotiamine is a lipid-soluble thiamine precursor which can transform to thiamine in vivo and subsequently be metabolized to thiamine monophosphate (TMP) and thiamine diphosphate (TDP). This study investigated the pharmacokinetic profiles of thiamine and its phosphorylated metabolites after single- and multiple-dose administration of benfotiamine in healthy Chinese volunteers, and assessed the bioavailability of orally benfotiamine administration compared to Thiamine hydrochloride. In addition, concentration of hippuric acid in urine which is produced in the transformation process of benfotiamine was determined. The results showed that thiamine and its phosphorylated metabolites exhibited different pharmacokinetic characteristics in plasma, blood and erythrocyte, and one-compartment model provided the best fit for pharmacokinetic profiles of thiamine. The transformation process of benfotiamine to thiamine produced large amount of hippuric acid. No accumulation of hippuric acid was observed after multiple-dose of benfotiamine. Compared to Thiamine hydrochloride, the bioavailability of thiamine in plasma and TDP in erythrocyte after oral administration of benfotiamine were 1147.3 +/- 490.3% and 195.8 +/- 33.8%, respectively. The absorption rate and extent of benfotiamine systemic availability of thiamine were significantly increased indicating higher bioavailability of thiamine from oral dose of benfotiamine compared to oral dose of Thiamine hydrochloride.


