WP1130Deubiquitinase (DUB) inhibitor, Cell permeable CAS# 856243-80-6 |
- NSC 687852 (b-AP15)
Catalog No.:BCC2389
CAS No.:1009817-63-3
- PR-619
Catalog No.:BCC3627
CAS No.:2645-32-1
- TCID
Catalog No.:BCC4449
CAS No.:30675-13-9
- IU1
Catalog No.:BCC2086
CAS No.:314245-33-5
- LDN 57444
Catalog No.:BCC2087
CAS No.:668467-91-2
- Vialinin A
Catalog No.:BCC2367
CAS No.:858134-23-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 856243-80-6 | SDF | Download SDF |
| PubChem ID | 11222830 | Appearance | Powder |
| Formula | C19H18BrN3O | M.Wt | 384.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Degrasyn | ||
| Solubility | DMSO : 100 mg/mL (260.23 mM; Need ultrasonic) | ||
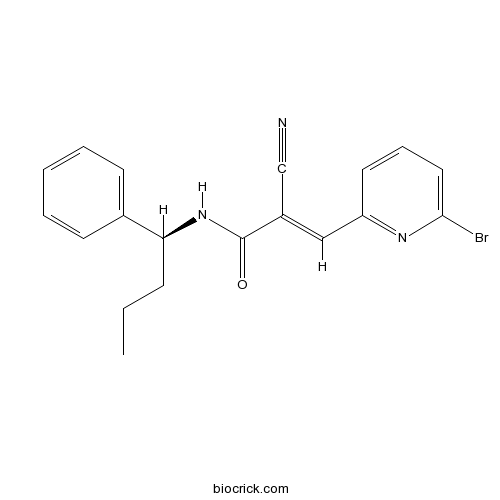
| Chemical Name | (E)-3-(6-bromopyridin-2-yl)-2-cyano-N-[(1S)-1-phenylbutyl]prop-2-enamide | ||
| SMILES | CCCC(C1=CC=CC=C1)NC(=O)C(=CC2=NC(=CC=C2)Br)C#N | ||
| Standard InChIKey | LIDOPKHSVQTSJY-VMEIHUARSA-N | ||
| Standard InChI | InChI=1S/C19H18BrN3O/c1-2-7-17(14-8-4-3-5-9-14)23-19(24)15(13-21)12-16-10-6-11-18(20)22-16/h3-6,8-12,17H,2,7H2,1H3,(H,23,24)/b15-12+/t17-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Degrasyn (WP1130) is a selective inhibitor of deubiquitinase (DUB: USP5, UCH-L1, USP9x, USP14, and UCH37). | |||||
| Targets | DUB | Bcr-Abl | ||||
| IC50 | 1.8 μM | |||||
| Cell experiment [1]: | |
| Cell lines | The human typical MCL cell lines (Mino, DB, Z-138, and JMP-1) |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 48 h; EC50=~1 μM |
| Applications | Degrasyn (WP1130) had superior inhibitory activity in the JAK/STAT pathway and the antiproliferative effect. These studies showed that cell growth suppression was seen in all three compounds(WP1130, WP1129 and WP1066 for all four cell lines, and the half maximal effective concentration (EC50) of degrasyn was lower than that of the other two compounds in all four MCL cell lines, suggesting that degrasyn is a more effective anti-tumor agent in MCL cells. |
| Animal experiment [1]: | |
| Animal models | Young SCID mice |
| Dosage form | 20 mg/kg; i.p. |
| Application | Useing a xenotransplant SCID mouse model of MCL. Degrasyn alone showed modest prolongation of survival compared with vehicle alone (P<0.105). In addition, normal control mice (n = 5) given treatment with degrasyn alone or in combination with bortezomib remained alive and showed no signs of body weight loss. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Pham L V, Tamayo A T, Li C, et al. Degrasyn potentiates the antitumor effects of bortezomib in mantle cell lymphoma cells in vitro and in vivo: therapeutic implications[J]. Molecular cancer therapeutics, 2010, 9(7): 2026-2036. | |

WP1130 Dilution Calculator

WP1130 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6023 mL | 13.0117 mL | 26.0234 mL | 52.0467 mL | 65.0584 mL |
| 5 mM | 0.5205 mL | 2.6023 mL | 5.2047 mL | 10.4093 mL | 13.0117 mL |
| 10 mM | 0.2602 mL | 1.3012 mL | 2.6023 mL | 5.2047 mL | 6.5058 mL |
| 50 mM | 0.052 mL | 0.2602 mL | 0.5205 mL | 1.0409 mL | 1.3012 mL |
| 100 mM | 0.026 mL | 0.1301 mL | 0.2602 mL | 0.5205 mL | 0.6506 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
WP1130, also known as degrasyn, is a second-generation tyrphostin derivative initially identified as a Janus-activated kinase (JAK)-signal transducer and activator of transcription (STAT) pathway inhibitor that reduces STAT activation stimulated by cytokines (such as IL-6 and IL-3) through the rapid down-regulation of upstream JAK kinases. WP1130 has also been identified as a selective deubiquitinating enzyme (DUB) inhibitor that directly inhibits DUB activity of USP9x, USP5, USP14 and UCH37 leading to rapid accumulation of polyubiquitinated proteins into juxtanuclear aggresomes and tumor cell apoptosis. WP1130-induced apoptosis and anti-proliferation in tumor cells have been implicated in the treatment of chronic myelogenous leukemia (CML), melanoma, glioblastoma and myeloproliferative disorders.
References:
[1]Kapuria V1, Peterson LF, Fang D, Bornmann WG, Talpaz M, Donato NJ. Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res. 2010 Nov 15;70(22):9265-76. doi: 10.1158/0008-5472.CAN-10-1530. Epub 2010 Nov 2.
[2]Bartholomeusz GA1, Talpaz M, Kapuria V, Kong LY, Wang S, Estrov Z, Priebe W, Wu J, Donato NJ. Activation of a novel Bcr/Abl destruction pathway by WP1130 induces apoptosis of chronic myelogenous leukemia cells. Blood. 2007 Apr 15;109(8):3470-8. Epub 2007 Jan 3.
- Temozolomide
Catalog No.:BCC4386
CAS No.:85622-93-1
- (2-Aminoethyl)phosphinic acid
Catalog No.:BCN1761
CAS No.:85618-16-2
- Gynuramine
Catalog No.:BCN2085
CAS No.:85611-43-4
- Zaltidine
Catalog No.:BCC2068
CAS No.:85604-00-8
- Kurarinol
Catalog No.:BCN3447
CAS No.:855746-98-4
- Rhodionin
Catalog No.:BCN1248
CAS No.:85571-15-9
- NBI-74330
Catalog No.:BCC4111
CAS No.:855527-92-3
- Safflor Yellow A
Catalog No.:BCN2408
CAS No.:85532-77-0
- PK 11195
Catalog No.:BCC6745
CAS No.:85532-75-8
- Eupatorin
Catalog No.:BCN4405
CAS No.:855-96-9
- 4-Chlorotestosterone acetate
Catalog No.:BCC8705
CAS No.:855-19-6
- Ajugamarin chlorohydrin
Catalog No.:BCN3664
CAS No.:85447-27-4
- Boc-Ala-NH2
Catalog No.:BCC3046
CAS No.:85642-13-3
- Curculigoside
Catalog No.:BCN4406
CAS No.:85643-19-2
- Laurycolactone A
Catalog No.:BCN3109
CAS No.:85643-76-1
- Laurycolactone B
Catalog No.:BCN3110
CAS No.:85643-77-2
- Mirtazapine
Catalog No.:BCC4923
CAS No.:85650-52-8
- Asenapine
Catalog No.:BCC2476
CAS No.:85650-56-2
- Setiptiline maleate
Catalog No.:BCC1946
CAS No.:85650-57-3
- Choline Fenofibrate
Catalog No.:BCC1478
CAS No.:856676-23-8
- CBiPES hydrochloride
Catalog No.:BCC7824
CAS No.:856702-40-4
- AM 114
Catalog No.:BCC3589
CAS No.:856849-35-9
- Tedizolid
Catalog No.:BCC1990
CAS No.:856866-72-3
- (-)-Blebbistatin
Catalog No.:BCC4375
CAS No.:856925-71-8
WP1130 increases doxorubicin sensitivity in hepatocellular carcinoma cells through usp9x-dependent p53 degradation.[Pubmed:25749422]
Cancer Lett. 2015 Jun 1;361(2):218-25.
Resistance to chemotherapeutic drugs is a major obstacle in hepatocellular carcinoma (HCC) therapy. However, the underlying mechanisms are not well understood. Recent evidence suggests that deubiquitinases (DUB) are key regulators in the mechanisms of cell proliferation, apoptosis and chemoresistance. The present study aimed to investigate whether WP1130, which inhibits activity of deubiquitinases, exerts synergistic cytotoxicity with doxorubicin in HCC and the underlying mechanisms. In the study, we found that Huh7, HepG2, and SNU387 HCC cells with p53 expression displayed enhanced response to the combination therapy compared with p53-deficient HCC cells (Hep3B) in the manner of inhibiting cell proliferation. Downregulation of p53 abolished the synergistic cytotoxicity of doxorubicin and WP1130 on HCC cells. Mechanistically, we found that combined treatment with WP1130 suppressed doxorubicin-mediated upregulation of p53 via promoting its ubiquitin-proteasome dependent degradation, whereas knockdown of DUB usp9x abolished this effect. Taken together, these results demonstrate that combined treatment with WP1130 sensitized HCC cells to doxorubicin via usp9x-depedent p53 degradation.
Activation of a novel Bcr/Abl destruction pathway by WP1130 induces apoptosis of chronic myelogenous leukemia cells.[Pubmed:17202319]
Blood. 2007 Apr 15;109(8):3470-8.
Imatinib mesylate (Gleevec) is effective therapy against Philadelphia chromosome-positive leukemia, but resistance develops in all phases of the disease. Bcr/Abl point mutations and other alterations reduce the kinase inhibitory activity of imatinib mesylate; thus, agents that target Bcr/Abl through unique mechanisms may be needed. Here we describe the activity of WP1130, a small molecule that specifically and rapidly down-regulates both wild-type and mutant Bcr/Abl protein without affecting bcr/abl gene expression in chronic myelogenous leukemia (CML) cells. Loss of Bcr/Abl protein correlated with the onset of apoptosis and reduced phosphorylation of Bcr/Abl substrates. WP1130 did not affect Hsp90/Hsp70 ratios within the cells and did not require the participation of the proteasomal pathway for loss of Bcr/Abl protein. WP1130 was more effective in reducing leukemic versus normal hematopoietic colony formation and strongly inhibited colony formation of cells derived from patients with T315I mutant Bcr/Abl-expressing CML in blast crisis. WP1130 suppressed the growth of K562 heterotransplanted tumors as well as both wild-type Bcr/Abl and T315I mutant Bcr/Abl-expressing BaF/3 cells transplanted into nude mice. Collectively, our results demonstrate that WP1130 reduces wild-type and T315I mutant Bcr/Abl protein levels in CML cells through a unique mechanism and may be useful in treating CML.
Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis.[Pubmed:21045142]
Cancer Res. 2010 Nov 15;70(22):9265-76.
Recent evidence suggests that several deubiquitinases (DUB) are overexpressed or activated in tumor cells and many contribute to the transformed phenotype. Agents with DUB inhibitory activity may therefore have therapeutic value. In this study, we describe the mechanism of action of WP1130, a small molecule derived from a compound with Janus-activated kinase 2 (JAK2) kinase inhibitory activity. WP1130 induces rapid accumulation of polyubiquitinated (K48/K63-linked) proteins into juxtanuclear aggresomes, without affecting 20S proteasome activity. WP1130 acts as a partly selective DUB inhibitor, directly inhibiting DUB activity of USP9x, USP5, USP14, and UCH37, which are known to regulate survival protein stability and 26S proteasome function. WP1130-mediated inhibition of tumor-activated DUBs results in downregulation of antiapoptotic and upregulation of proapoptotic proteins, such as MCL-1 and p53. Our results show that chemical modification of a previously described JAK2 inhibitor results in the unexpected discovery of a novel DUB inhibitor with a unique antitumor mechanism.
Deubiquitinase inhibition by WP1130 leads to ULK1 aggregation and blockade of autophagy.[Pubmed:26207339]
Autophagy. 2015;11(9):1458-70.
Autophagy represents an intracellular degradation process which is involved in both regular cell homeostasis and disease settings. In recent years, the molecular machinery governing this process has been elucidated. The ULK1 kinase complex consisting of the serine/threonine protein kinase ULK1 and the adapter proteins ATG13, RB1CC1, and ATG101, is centrally involved in the regulation of autophagy initiation. This complex is in turn regulated by the activity of different nutrient- or energy-sensing kinases, including MTOR, AMPK, and AKT. However, next to phosphorylation processes it has been suggested that ubiquitination of ULK1 positively influences ULK1 function. Here we report that the inhibition of deubiquitinases by the compound WP1130 leads to increased ULK1 ubiquitination, the transfer of ULK1 to aggresomes, and the inhibition of ULK1 activity. Additionally, WP1130 can block the autophagic flux. Thus, treatment with WP1130 might represent an efficient tool to inhibit the autophagy-initiating ULK1 complex and autophagy.


