AM 11420S proteasome inhibitor CAS# 856849-35-9 |
- MLN2238
Catalog No.:BCC2092
CAS No.:1072833-77-2
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- Salinosporamide A (NPI-0052, Marizomib)
Catalog No.:BCC2094
CAS No.:437742-34-2
- Gliotoxin
Catalog No.:BCN3894
CAS No.:67-99-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 856849-35-9 | SDF | Download SDF |
| PubChem ID | 11639443 | Appearance | Powder |
| Formula | C20H21B2NO5 | M.Wt | 377.01 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Proteasome Inhibitor IX, AM114; | ||
| Solubility | Soluble to 10 mM in DMSO | ||
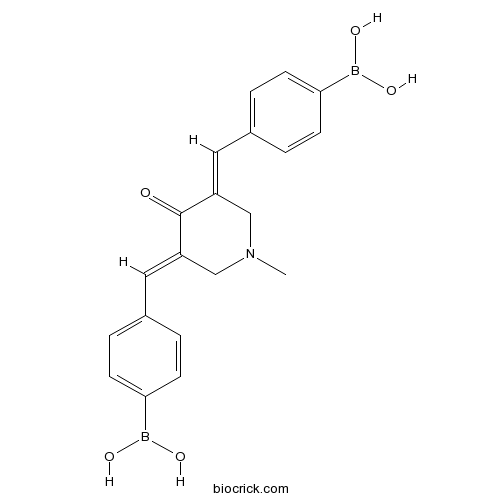
| Chemical Name | [4-[(E)-[(5E)-5-[(4-boronophenyl)methylidene]-1-methyl-4-oxopiperidin-3-ylidene]methyl]phenyl]boronic acid | ||
| SMILES | B(C1=CC=C(C=C1)C=C2CN(CC(=CC3=CC=C(C=C3)B(O)O)C2=O)C)(O)O | ||
| Standard InChIKey | SRPIKXGUPAKTIZ-OTYYAQKOSA-N | ||
| Standard InChI | InChI=1S/C20H21B2NO5/c1-23-12-16(10-14-2-6-18(7-3-14)21(25)26)20(24)17(13-23)11-15-4-8-19(9-5-15)22(27)28/h2-11,25-28H,12-13H2,1H3/b16-10+,17-11+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of the chymotrypsin-like activity of the 20S proteasome (IC50 ~ 1 μM). Displays anticancer activity; inhibits cell growth in human colon cancer HCT116 p53+/+ cells (IC50 = 1.49 μM). |

AM 114 Dilution Calculator

AM 114 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6524 mL | 13.2622 mL | 26.5245 mL | 53.049 mL | 66.3112 mL |
| 5 mM | 0.5305 mL | 2.6524 mL | 5.3049 mL | 10.6098 mL | 13.2622 mL |
| 10 mM | 0.2652 mL | 1.3262 mL | 2.6524 mL | 5.3049 mL | 6.6311 mL |
| 50 mM | 0.053 mL | 0.2652 mL | 0.5305 mL | 1.061 mL | 1.3262 mL |
| 100 mM | 0.0265 mL | 0.1326 mL | 0.2652 mL | 0.5305 mL | 0.6631 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CBiPES hydrochloride
Catalog No.:BCC7824
CAS No.:856702-40-4
- Choline Fenofibrate
Catalog No.:BCC1478
CAS No.:856676-23-8
- Setiptiline maleate
Catalog No.:BCC1946
CAS No.:85650-57-3
- Asenapine
Catalog No.:BCC2476
CAS No.:85650-56-2
- Mirtazapine
Catalog No.:BCC4923
CAS No.:85650-52-8
- Laurycolactone B
Catalog No.:BCN3110
CAS No.:85643-77-2
- Laurycolactone A
Catalog No.:BCN3109
CAS No.:85643-76-1
- Curculigoside
Catalog No.:BCN4406
CAS No.:85643-19-2
- Boc-Ala-NH2
Catalog No.:BCC3046
CAS No.:85642-13-3
- WP1130
Catalog No.:BCC3686
CAS No.:856243-80-6
- Temozolomide
Catalog No.:BCC4386
CAS No.:85622-93-1
- (2-Aminoethyl)phosphinic acid
Catalog No.:BCN1761
CAS No.:85618-16-2
- Tedizolid
Catalog No.:BCC1990
CAS No.:856866-72-3
- (-)-Blebbistatin
Catalog No.:BCC4375
CAS No.:856925-71-8
- alpha-Conidendrin
Catalog No.:BCN4407
CAS No.:85699-62-3
- (3S,3'R,8R,9R,9As)-8-methoxy-3'-methyl-3-[(2S,4S)-4-methyl-5-oxooxolan-2-yl]spiro[1,2,3,5,6,7,8,9a-octahydropyrrolo[1,2-a]azepine-9,5'-oxolane]-2'-one
Catalog No.:BCC9250
CAS No.:85700-47-6
- Scopine HCl
Catalog No.:BCC4940
CAS No.:85700-55-6
- WP1066
Catalog No.:BCC2194
CAS No.:857064-38-1
- TMC353121
Catalog No.:BCC2004
CAS No.:857066-90-1
- PF 915275
Catalog No.:BCC7631
CAS No.:857290-04-1
- 3-Hydroxysarpagine
Catalog No.:BCN4566
CAS No.:857297-90-6
- Isoflavidinin
Catalog No.:BCN7604
CAS No.:85734-02-7
- Retaspimycin
Catalog No.:BCC1889
CAS No.:857402-23-4
- IPI-504 (Retaspimycin hydrochloride)
Catalog No.:BCC2126
CAS No.:857402-63-2
Comments on "Intraspecific and geographic variation of West Indian manatee (Trichechus manatus spp.) vocalizations" [J. Acoust. Soc. Am. 114, 66-69 (2003)].[Pubmed:16838493]
J Acoust Soc Am. 2006 Jun;119(6):3537.
This letter concerns the paper "Intraspecific and geographic variation of West Indian manatee (Trichechus manatus spp.) vocalizations" [Nowacek et al., J. Acoust. Soc. Am. 114, 66-69 (2003)]. The purpose here is to correct the fundamental frequency range and information on intraindividual variation in the vocalizations of Amazonian manatees reported by Nowacek et al. (2003) in citing the paper "Signature information and individual recognition in the isolation calls of Amazonian manatees, Trichechus inunguis (Mammalia: Sirenia)" [Sousa-Lima et al., Anim. Behav. 63, 301-310 (2002)].
A boronic-chalcone derivative exhibits potent anticancer activity through inhibition of the proteasome.[Pubmed:16636137]
Mol Pharmacol. 2006 Jul;70(1):426-33.
Chalcones and their derivatives have been shown to have potent anticancer activity. However, the exact mechanisms of cytotoxic activity remain to be established. In this study, we have evaluated a series of boronic chalcones for their anticancer activity and mechanisms of action. Among the eight chalcone derivatives tested, 3,5-bis-(4-boronic acid-benzylidene)-1-methyl-piperidin-4-one (AM114) exhibited most potent growth inhibitory activity with IC50 values of 1.5 and 0.6 microM in 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and colony formation assay, respectively. The cytotoxic activity of AM114 was shown to be associated with the accumulation of p53 and p21 proteins and induction of apoptosis. Mechanistic studies showed that AM114 treatment inhibited the chymotrypsin-like activity of the 20S proteasome in vitro, leading to a significant accumulation of ubiquitinated p53 and other cellular proteins in whole cells. In vitro studies showed that AM114 did not significantly disrupt the interaction of p53 and murine double minute 2 protein. It is noteworthy that AM114 as a single agent was preferentially toxic to cells with wild-type p53 expression, whereas combination of this compound with ionizing radiation (IR) significantly enhanced the cell-killing activity of IR in both wild-type p53 and p53-null cells. Together, these results indicate that the boronic chalcone derivative AM114 induces significant cytotoxic effect in cancer cells through the inhibition of the cellular proteasome and provide a rationale for the further development of this class of compounds as novel cancer chemotherapeutic agents.


