PF 915275CAS# 857290-04-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 857290-04-1 | SDF | Download SDF |
| PubChem ID | 23725123 | Appearance | Powder |
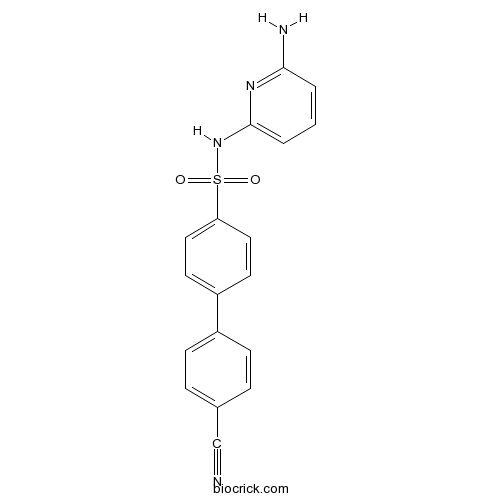
| Formula | C18H14N4O2S | M.Wt | 350.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 31 mg/mL (88.47 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-(6-aminopyridin-2-yl)-4-(4-cyanophenyl)benzenesulfonamide | ||
| SMILES | C1=CC(=NC(=C1)NS(=O)(=O)C2=CC=C(C=C2)C3=CC=C(C=C3)C#N)N | ||
| Standard InChIKey | ZESFDAKNYJQYKO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H14N4O2S/c19-12-13-4-6-14(7-5-13)15-8-10-16(11-9-15)25(23,24)22-18-3-1-2-17(20)21-18/h1-11H,(H3,20,21,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) inhibitor (Ki = 2.3 nM) that displays little activity at 11βHSD2 (1.5% inhibition at 10 μM). Inhibits the conversion of prednisone to prednisolone in human hepatocytes in vitro (EC50 = 15 nM) and has antidiabetic activity in vivo. Orally active. |

PF 915275 Dilution Calculator

PF 915275 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.854 mL | 14.2698 mL | 28.5396 mL | 57.0793 mL | 71.3491 mL |
| 5 mM | 0.5708 mL | 2.854 mL | 5.7079 mL | 11.4159 mL | 14.2698 mL |
| 10 mM | 0.2854 mL | 1.427 mL | 2.854 mL | 5.7079 mL | 7.1349 mL |
| 50 mM | 0.0571 mL | 0.2854 mL | 0.5708 mL | 1.1416 mL | 1.427 mL |
| 100 mM | 0.0285 mL | 0.1427 mL | 0.2854 mL | 0.5708 mL | 0.7135 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PF-915275 is a potent and selective inhibitor of human 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) (Ki < 1 nM) with good preclinical pharmacokinetic properties. IC50 value: < 1 nM (Ki)[2] Target: 11βHSD1 in vitro: PF-915275 maintains potency in our cellular assay against human 11βHSD1 (HEK293, EC50 = 5 nM) and is selective against human 11βHSD2 (HEK293, 1.5% inhibition 10 μM). PF-915275 displays only weak affinity for the rodent choline transporter (Ki = 9.6 μM) and the hamster melatonin MT3 receptor (Ki = 9.6 μM) in the Cerep Bioprint screening panel. PF-915275 has good in vitro pharmacokinetic properties. In particular, PF-915275 is categorized as a low clearance compound (liver microsome assays) with high permeability (Caco2 assay). [2] in vivo: As a prelude to in vivo studies with PF-915275, the rat pharmacokinetic properties of this compound were determined. PF-915275 has an excellent pharmacokinetic profile characterized by low clearance, long half-life and good oral bioavailability. [2]
References:
[1]. Bhat BG, et al. Demonstration of proof of mechanism and pharmacokinetics and pharmacodynamic relationship with 4'-cyano-biphenyl-4-sulfonic acid (6-amino-pyridin-2-yl)-amide (PF-915275), an inhibitor of 11 -hydroxysteroid dehydrogenase type 1, in cynomolg
[2]. Siu M, et al. N-(Pyridin-2-yl) arylsulfonamide inhibitors of 11beta-hydroxysteroid dehydrogenase type 1: Discovery of PF-915275. Bioorg Med Chem Lett. 2009 Jul 1;19(13):3493-7.
- TMC353121
Catalog No.:BCC2004
CAS No.:857066-90-1
- WP1066
Catalog No.:BCC2194
CAS No.:857064-38-1
- Scopine HCl
Catalog No.:BCC4940
CAS No.:85700-55-6
- (3S,3'R,8R,9R,9As)-8-methoxy-3'-methyl-3-[(2S,4S)-4-methyl-5-oxooxolan-2-yl]spiro[1,2,3,5,6,7,8,9a-octahydropyrrolo[1,2-a]azepine-9,5'-oxolane]-2'-one
Catalog No.:BCC9250
CAS No.:85700-47-6
- alpha-Conidendrin
Catalog No.:BCN4407
CAS No.:85699-62-3
- (-)-Blebbistatin
Catalog No.:BCC4375
CAS No.:856925-71-8
- Tedizolid
Catalog No.:BCC1990
CAS No.:856866-72-3
- AM 114
Catalog No.:BCC3589
CAS No.:856849-35-9
- CBiPES hydrochloride
Catalog No.:BCC7824
CAS No.:856702-40-4
- Choline Fenofibrate
Catalog No.:BCC1478
CAS No.:856676-23-8
- Setiptiline maleate
Catalog No.:BCC1946
CAS No.:85650-57-3
- Asenapine
Catalog No.:BCC2476
CAS No.:85650-56-2
- 3-Hydroxysarpagine
Catalog No.:BCN4566
CAS No.:857297-90-6
- Isoflavidinin
Catalog No.:BCN7604
CAS No.:85734-02-7
- Retaspimycin
Catalog No.:BCC1889
CAS No.:857402-23-4
- IPI-504 (Retaspimycin hydrochloride)
Catalog No.:BCC2126
CAS No.:857402-63-2
- K-252c
Catalog No.:BCC3706
CAS No.:85753-43-1
- AT7867
Catalog No.:BCC2536
CAS No.:857531-00-1
- PSN632408
Catalog No.:BCC5408
CAS No.:857652-30-3
- Longistylumphylline A
Catalog No.:BCN4408
CAS No.:857672-34-5
- Alstolenine
Catalog No.:BCN4808
CAS No.:85769-33-1
- Polygalaxanthone XI
Catalog No.:BCN7366
CAS No.:857859-82-6
- Motesanib Diphosphate (AMG-706)
Catalog No.:BCC2477
CAS No.:857876-30-3
- 11,12-Di-O-acetyltenacigenin B
Catalog No.:BCN4565
CAS No.:857897-01-9
Demonstration of proof of mechanism and pharmacokinetics and pharmacodynamic relationship with 4'-cyano-biphenyl-4-sulfonic acid (6-amino-pyridin-2-yl)-amide (PF-915275), an inhibitor of 11 -hydroxysteroid dehydrogenase type 1, in cynomolgus monkeys.[Pubmed:17921190]
J Pharmacol Exp Ther. 2008 Jan;324(1):299-305.
Glucocorticoids, through activation of the glucocorticoid receptor (GR), regulate hepatic gluconeogenesis. Elevated hepatic expression and activity of 11beta-hydroxysteroid dehydrogenase type 1 (11betaHSD1) play a key role in ligand-induced activation of the GR through the production of cortisol. Evidence from genetically modified mice suggests that inhibition of 11betaHSD1 might be a therapeutic approach to treat the metabolic syndrome. We have identified a potent 11betaHSD1 inhibitor, 4'-cyano-biphenyl-4-sulfonic acid (6-amino-pyridin-2-yl)-amide (PF-915275), that is selective for the primate and human enzymes. The objective of this study was to demonstrate target inhibition with PF-915275 and to quantify the relationship between target inhibition and drug exposure in monkeys. We characterized the ability of PF-915275 to inhibit the conversion of prednisone, a synthetic cortisone analog that can be distinguished from the endogenous substrate cortisone, enabling a direct measure of substrate to product conversion without the complication of feedback. Adult cynomolgus monkeys were administered either vehicle or various doses of PF-915275 followed by a 10-mg/kg dose of prednisone. Prednisone conversion to prednisolone and the concentrations of PF-915275 were measured by liquid chromatography/tandem mass spectrometry. PF-915275 dose-dependently inhibited 11betaHSD1-mediated conversion of prednisone to prednisolone, with a maximum of 87% inhibition at a 3-mg/kg dose. An exposure-response relationship was demonstrated, with an estimated EC(50) of 391 nM (total) and 17 nM (free). Insulin levels were also reduced in a dose-related manner. These results should enable the development of a biomarker for evaluating target modulation in humans that will aid in identifying 11betaHSD1 inhibitors to treat diabetes and other related metabolic diseases.
N-(Pyridin-2-yl) arylsulfonamide inhibitors of 11beta-hydroxysteroid dehydrogenase type 1: Discovery of PF-915275.[Pubmed:19473839]
Bioorg Med Chem Lett. 2009 Jul 1;19(13):3493-7.
N-(Pyridin-2-yl) arylsulfonamides are identified as inhibitors of 11beta-hydroxysteroid dehydrogenase type 1 (11betaHSD1), an enzyme that catalyzes the reduction of the glucocorticoid cortisone to cortisol. Dysregulation of glucocorticoids has been implicated in the pathogenesis of diabetes and the metabolic syndrome. In this Letter, we present the development of an initial lead to an efficient ligand with improved physiochemical properties using a deletion strategy. This strategy allowed for further optimization of potency leading to the discovery of the clinical candidate PF-915275.
Modulation of 11beta-hydroxysteroid dehydrogenase (11betaHSD) activity biomarkers and pharmacokinetics of PF-00915275, a selective 11betaHSD1 inhibitor.[Pubmed:17986636]
J Clin Endocrinol Metab. 2008 Feb;93(2):550-6.
CONTEXT: 11beta-Hydroxysteroid dehydrogenase type 1 (11betaHSD1) is a promising target for the treatment of type 2 diabetes mellitus. 11betaHSD1 catalyzes the intracrine conversion of inactive cortisone to the active glucocorticoid cortisol. OBJECTIVE: Demonstrating inhibition of 11betaHSD1 is challenging because there is no accessible way to directly assess the enzyme activity in vivo. Thus, it was proposed to assess the enzyme activity, in an indirect fashion, using two biomarker methods: the prednisolone generation study (conversion of oral prednisone to prednisolone in plasma) and the ratio of cortisol and cortisone metabolites in urine. DESIGN: This was a phase 1, double-blind, placebo-controlled, randomized, multiple-dose study. SETTING: The study was conducted in a clinical research unit. PARTICIPANTS: Sixty healthy adult volunteers participated in the study. INTERVENTION: Oral doses of PF-00915275 (0.3-15 mg) and prednisone (10 mg) were administered during the study. MAIN OUTCOME MEASURES: Safety, tolerability, pharmacokinetics, and pharmacodynamics of PF-00915275, a selective 11betaHSD1 inhibitor, were measured. RESULTS: Overall, multiple oral doses of PF-00915275 were safe and well tolerated. After oral administration, PF-00915275 was rapidly absorbed, slowly eliminated, and generally displayed dose-proportional increases in exposure. At the 15-mg dose, mean exposure to prednisolone was reduced by 37%, and there was a dose-dependent fall in the 5alpha-tetrahydrocortisol + 5beta-tetrahydrocortisol to tetrahydrocortisone ratio with maximum inhibition of 26% after 14 d. The urinary free cortisol to urinary free cortisone ratio, an indicator of 11betaHSD2 inhibition, did not change. CONCLUSION: PF-00915275 was safe at all doses tested. The results of the prednisolone generation test and the urinary metabolite ratios confirm that PF-00915275 is a selective 11betaHSD1 inhibitor.


