Motesanib Diphosphate (AMG-706)VEGFR/ PDGFR/c-Kit/Ret inhibitor CAS# 857876-30-3 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Docetaxel Trihydrate
Catalog No.:BCC1535
CAS No.:148408-66-6
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
- 7-Xylosyltaxol
Catalog No.:BCN5341
CAS No.:90332-66-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 857876-30-3 | SDF | Download SDF |
| PubChem ID | 16097729 | Appearance | Powder |
| Formula | C22H29N5O9P2 | M.Wt | 569.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Motesanib; AMG 706 | ||
| Solubility | DMSO : ≥ 110 mg/mL (193.17 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-(3,3-dimethyl-1,2-dihydroindol-6-yl)-2-(pyridin-4-ylmethylamino)pyridine-3-carboxamide;phosphoric acid | ||
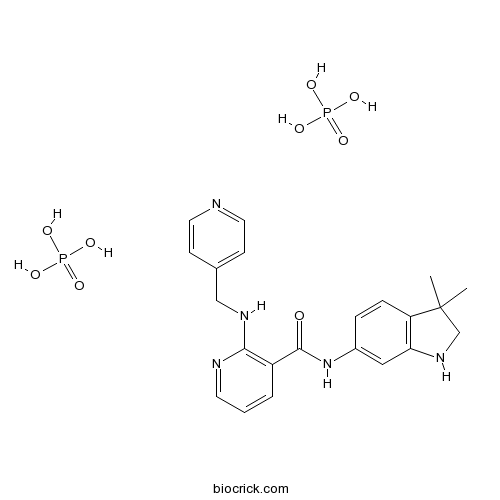
| SMILES | CC1(CNC2=C1C=CC(=C2)NC(=O)C3=C(N=CC=C3)NCC4=CC=NC=C4)C.OP(=O)(O)O.OP(=O)(O)O | ||
| Standard InChIKey | ONDPWWDPQDCQNJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H23N5O.2H3O4P/c1-22(2)14-26-19-12-16(5-6-18(19)22)27-21(28)17-4-3-9-24-20(17)25-13-15-7-10-23-11-8-15;2*1-5(2,3)4/h3-12,26H,13-14H2,1-2H3,(H,24,25)(H,27,28);2*(H3,1,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Motesanib Diphosphate is an orally bioavailable inhibitor of multiple-receptor tyrosine kinases. | ||||||
| Targets | VEGFR | PDGFR | kit | Ret | |||

Motesanib Diphosphate (AMG-706) Dilution Calculator

Motesanib Diphosphate (AMG-706) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7561 mL | 8.7806 mL | 17.5611 mL | 35.1222 mL | 43.9028 mL |
| 5 mM | 0.3512 mL | 1.7561 mL | 3.5122 mL | 7.0244 mL | 8.7806 mL |
| 10 mM | 0.1756 mL | 0.8781 mL | 1.7561 mL | 3.5122 mL | 4.3903 mL |
| 50 mM | 0.0351 mL | 0.1756 mL | 0.3512 mL | 0.7024 mL | 0.8781 mL |
| 100 mM | 0.0176 mL | 0.0878 mL | 0.1756 mL | 0.3512 mL | 0.439 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Motesanib Diphosphate is a diphosphate form of motesanib. It is a potent ATP-competitive inhibitor of VEGFR1/2/3 with IC50 of 2 nM/3 nM/6 nM, respectively [1].
Vascular endothelial growth factor (VEGF) is an important signaling protein involved in both vasculogenesis (the formation of the circulatory system) and angiogenesis (the growth of blood vessels from pre-existing vasculature). Motesanib (AMG 706) is an orally administered small molecule belonging to angiokinase inhibitor class which acts as an antagonist of VEGF receptors, platelet-derived growth factor receptors, and stem cell factor receptors.
In vitro: Motesanib diphosphate has broad activity against the human VEGFR family, and displays >1000 selectivity against EGFR, Src, and p38 kinase. Motesanib Diphosphate significantly inhibits VEGF-induced cellular proliferation of HUVECs with an IC50 of 10 nM, while displaying little effect at bFGF-induced proliferation with an IC50 of >3,000 nM. Motesanib Diphosphate also potently inhibits PDGF-induced proliferation and SCF-induced c-kit phosphorylation with IC50 of 207 nM and 37 nM, respectively, but not effective against the EGF-induced EGFR phosphorylation and cell viability of A431 cells [1]. Althouth displaying little antiproliferative activity on cell growth of HUVECs alone, Motesanib diphosphate treatment significantly sensitizes the cells to fractionated radiation [2].
In vivo: Oral administration of AMG 706 potently inhibited VEGF-induced angiogenesis in the rat corneal model and induced regression of established A431 xenografts. AMG 706 was well tolerated and had no significant effects o AMG 706n body weight or on the general health of the animals. Histologic analysis of tumor xenografts from AMG 706–treated animals revealed an increase in endothelial apoptosis and a reduction in blood vessel area that preceded an increase in tumor cell apoptosis. In summary, AMG 706 is an orally bioavailable, well-tolerated multikinase inhibitor that is presently under clinical investigation for the treatment of human malignancies [1].
Clinical trial: Motesanib was originally investigated for effectiveness against advanced nonsquamous non-small-cell lung cancer (NSCLC), with Phase II trials indicating an effectiveness comparable to bevacizumab when they were both used in combination with paclitaxel/carboplatin. However a later and more detailed Phase III trial failed to show any benefit for the treatment of NSCLC. A second Phase III trial was started in 2012, which focused on patients from Asian backgrounds (performed on the bases of subgroup analysis) however this also failed to meet its primary endpoint. The drug has undergone a Phase II evaluation as first-line therapy for breast cancer however this study found no evidence to support further investigation. Phase II testing against persistent or recurrent ovarian, fallopian tube and primary peritoneal carcinomas was also unsuccessful. There have also been 2 separate Phase II clinical trials for thyroid cancer which have both shown promising results (http://en.wikipedia.org/wiki/Motesanib).
References:
[1] Polverino A, Coxon A, Starnes C, et al. AMG 706, an oral, multikinase inhibitor that selectively targets vascular endothelial growth factor, platelet-derived growth factor, and kit receptors, potently inhibits angiogenesis and induces regression in tumor xenografts. Cancer Res. 2006;66(17):8715-21.
[2] Kruser TJ1 Wheeler DL, Armstrong EA, Iida M, Kozak KR, van der Kogel AJ, Bussink J, Coxon A, Polverino A, Harari PM. Augmentation of radiation response by motesanib, a multikinase inhibitor that targets vascular endothelial growth factor receptors. Clin Cancer Res. 2010;16(14):3639-47.
- Polygalaxanthone XI
Catalog No.:BCN7366
CAS No.:857859-82-6
- Alstolenine
Catalog No.:BCN4808
CAS No.:85769-33-1
- Longistylumphylline A
Catalog No.:BCN4408
CAS No.:857672-34-5
- PSN632408
Catalog No.:BCC5408
CAS No.:857652-30-3
- AT7867
Catalog No.:BCC2536
CAS No.:857531-00-1
- K-252c
Catalog No.:BCC3706
CAS No.:85753-43-1
- IPI-504 (Retaspimycin hydrochloride)
Catalog No.:BCC2126
CAS No.:857402-63-2
- Retaspimycin
Catalog No.:BCC1889
CAS No.:857402-23-4
- Isoflavidinin
Catalog No.:BCN7604
CAS No.:85734-02-7
- 3-Hydroxysarpagine
Catalog No.:BCN4566
CAS No.:857297-90-6
- PF 915275
Catalog No.:BCC7631
CAS No.:857290-04-1
- TMC353121
Catalog No.:BCC2004
CAS No.:857066-90-1
- 11,12-Di-O-acetyltenacigenin B
Catalog No.:BCN4565
CAS No.:857897-01-9
- (-)-Quinpirole hydrochloride
Catalog No.:BCC6917
CAS No.:85798-08-9
- 3-O-Benzyl estrone
Catalog No.:BCC8638
CAS No.:858-98-0
- Vialinin A
Catalog No.:BCC2367
CAS No.:858134-23-3
- Marsdenoside F
Catalog No.:BCN4564
CAS No.:858360-61-9
- 1,3-Dipropyl-8-phenylxanthine
Catalog No.:BCC6664
CAS No.:85872-53-3
- prim-O-Glucosylangelicain
Catalog No.:BCN4409
CAS No.:85889-15-2
- Lincomycin hydrochloride
Catalog No.:BCC9011
CAS No.:859-18-7
- 6-Iodonordihydrocapsaicin
Catalog No.:BCC5860
CAS No.:859171-97-4
- 4-O-Methylhonokiol
Catalog No.:BCN8474
CAS No.:68592-15-4
- Acetyl meldrum's acid
Catalog No.:BCC8805
CAS No.:85920-63-4
- AGN 205728
Catalog No.:BCC5418
CAS No.:859498-05-8
Effect of coadministration of ketoconazole, a strong CYP3A4 inhibitor, on pharmacokinetics and tolerability of motesanib diphosphate (AMG 706) in patients with advanced solid tumors.[Pubmed:18574557]
Invest New Drugs. 2008 Oct;26(5):455-62.
Motesanib diphosphate is a novel angiogenesis inhibitor selectively targeting vascular endothelial growth factor receptors 1, 2, and 3; platelet-derived growth factor receptor and stem cell factor receptor. The purpose of this phase 1b, drug-drug interaction study was to investigate the effect of ketoconazole, a strong inhibitor of the cytochrome P450 3A4 isoenzyme, on the pharmacokinetics and tolerability of motesanib diphosphate. Fourteen patients with advanced solid tumors refractory to standard treatment were enrolled and received motesanib diphosphate 50 mg once daily from day 1 through 15. Patients were randomized to receive a single oral dose of ketoconazole 400 mg either on day 8 (Sequence 1; n = 7) or day 15 (Sequence 2; n = 7), while pharmacokinetic samples were collected. After completion of this part (day 16), 13 patients received an escalated once-daily dose of motesanib diphosphate 125 mg. Evaluable pharmacokinetic data (n = 12) suggest that ketoconazole modestly increased motesanib exposure. The motesanib area under the concentration-time curve (AUC) from 0 to 24 h increased by 86% (90% CI, 1.50-2.29; P < 0.001) and the maximum plasma concentration (C (max)) by 35% (90% CI, 1.12-1.64; P = 0.02), compared with motesanib diphosphate administration alone. The tolerability profile (with or without ketoconazole coadministration) was consistent with that from other motesanib diphosphate monotherapy studies. Treatment-related adverse events were mild to moderate and commonly included fatigue (50% of patients), hypertension (43%), diarrhea (21%), dizziness (14%), paresthesia (14%), and vomiting (14%). Hypertension was the most common related grade 3 event (21%). No grade 4 or 5 treatment-related adverse events occurred.


