(-)-Quinpirole hydrochlorideSelective D2-like agonist CAS# 85798-08-9 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

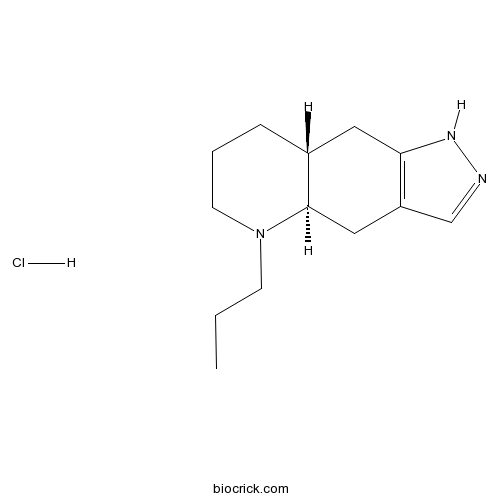
| Cas No. | 85798-08-9 | SDF | Download SDF |
| PubChem ID | 55397 | Appearance | Powder |
| Formula | C13H22ClN3 | M.Wt | 255.79 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 31.25 mg/mL (122.17 mM; Need ultrasonic) | ||
| Chemical Name | (4aR,8aR)-5-propyl-1,4,4a,6,7,8,8a,9-octahydropyrazolo[3,4-g]quinoline;hydrochloride | ||
| SMILES | CCCN1CCCC2C1CC3=C(C2)NN=C3.Cl | ||
| Standard InChIKey | HJHVRVJTYPKTHX-HTMVYDOJSA-N | ||
| Standard InChI | InChI=1S/C13H21N3.ClH/c1-2-5-16-6-3-4-10-7-12-11(8-13(10)16)9-14-15-12;/h9-10,13H,2-8H2,1H3,(H,14,15);1H/t10-,13-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective dopamine D2 receptor agonist (Ki values are 4.8, ~24, ~30 and 1900 nM at D2, D3, D4 and D1 receptors respectively). |

(-)-Quinpirole hydrochloride Dilution Calculator

(-)-Quinpirole hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9095 mL | 19.5473 mL | 39.0946 mL | 78.1891 mL | 97.7364 mL |
| 5 mM | 0.7819 mL | 3.9095 mL | 7.8189 mL | 15.6378 mL | 19.5473 mL |
| 10 mM | 0.3909 mL | 1.9547 mL | 3.9095 mL | 7.8189 mL | 9.7736 mL |
| 50 mM | 0.0782 mL | 0.3909 mL | 0.7819 mL | 1.5638 mL | 1.9547 mL |
| 100 mM | 0.0391 mL | 0.1955 mL | 0.3909 mL | 0.7819 mL | 0.9774 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 11,12-Di-O-acetyltenacigenin B
Catalog No.:BCN4565
CAS No.:857897-01-9
- Motesanib Diphosphate (AMG-706)
Catalog No.:BCC2477
CAS No.:857876-30-3
- Polygalaxanthone XI
Catalog No.:BCN7366
CAS No.:857859-82-6
- Alstolenine
Catalog No.:BCN4808
CAS No.:85769-33-1
- Longistylumphylline A
Catalog No.:BCN4408
CAS No.:857672-34-5
- PSN632408
Catalog No.:BCC5408
CAS No.:857652-30-3
- AT7867
Catalog No.:BCC2536
CAS No.:857531-00-1
- K-252c
Catalog No.:BCC3706
CAS No.:85753-43-1
- IPI-504 (Retaspimycin hydrochloride)
Catalog No.:BCC2126
CAS No.:857402-63-2
- Retaspimycin
Catalog No.:BCC1889
CAS No.:857402-23-4
- Isoflavidinin
Catalog No.:BCN7604
CAS No.:85734-02-7
- 3-Hydroxysarpagine
Catalog No.:BCN4566
CAS No.:857297-90-6
- 3-O-Benzyl estrone
Catalog No.:BCC8638
CAS No.:858-98-0
- Vialinin A
Catalog No.:BCC2367
CAS No.:858134-23-3
- Marsdenoside F
Catalog No.:BCN4564
CAS No.:858360-61-9
- 1,3-Dipropyl-8-phenylxanthine
Catalog No.:BCC6664
CAS No.:85872-53-3
- prim-O-Glucosylangelicain
Catalog No.:BCN4409
CAS No.:85889-15-2
- Lincomycin hydrochloride
Catalog No.:BCC9011
CAS No.:859-18-7
- 6-Iodonordihydrocapsaicin
Catalog No.:BCC5860
CAS No.:859171-97-4
- 4-O-Methylhonokiol
Catalog No.:BCN8474
CAS No.:68592-15-4
- Acetyl meldrum's acid
Catalog No.:BCC8805
CAS No.:85920-63-4
- AGN 205728
Catalog No.:BCC5418
CAS No.:859498-05-8
- GR 125487 sulfamate
Catalog No.:BCC7142
CAS No.:859502-43-5
- FIT
Catalog No.:BCC7082
CAS No.:85951-63-9
Quinpirole hydrochloride, a potential anti-parkinsonism drug.[Pubmed:3309709]
Neuropharmacology. 1987 Aug;26(8):1031-6.
Quinpirole hydrochloride, a putative dopamine agonist, was investigated in animal models of central dopaminergic activity, to evaluate its possible role in the treatment of Parkinson's disease. The drug induced stereotyped sniffing in rats but, unlike apomorphine, did not produce a maximal behavioural response (stereotyped gnawing). Pretreatment with neuroleptics blocked the stereotypy induced by quinpirole. Quinpirole reversed the effects of reserpine and alpha-methyl-paratyrosine, caused dose-dependent contralateral rotations in rats with unilateral lesions of the substantia nigra induced by 6-hydroxydopamine and induced vomiting in dogs. Small doses of quinpirole decreased locomotor activity, an effect presumably mediated by pre-synaptic autoreceptors. Quinpirole bound to D2 dopamine receptors in the striatum of the rat. The chronic injection of both subthreshold and suprathreshold doses, failed to induce behavioral supersensitivity. These data indicate that quinpirole can stimulate central dopaminergic receptors, and that it is a partial agonist with direct-acting properties. Quinpirole differs from other dopaminergic drugs and may be useful for the therapy of Parkinson's disease.
The stimulatory and inhibitory effects of quinpirole hydrochloride, D2-dopamine receptor agonist, on secretion of prolactin as assessed by the reverse hemolytic plaque assay.[Pubmed:8100980]
Life Sci. 1993;53(4):305-13.
Recent findings indicate that low concentrations of dopamine (DA) stimulate the secretion of prolactin (PRL) in vitro. In this study, we found that low concentrations of the highly-specific DA D2 receptor agonist, quinpirole hydrochloride (LY) stimulate PRL secretion in female rats, assessed by reverse hemolytic plaque assay. Low concentrations of LY (10(-12), 10(-10) M) increased the mean plaque area and increased the fraction of lactotrophs forming large plaques. On the other hand, higher concentrations of LY (10(-8), 10(-6) M) reduced the mean plaque size. Treatment of cells with 10(-6) M LY produced a unimodal distribution of small plaques. Low concentrations of LY (10(-12), 10(-10) M) with TRH (10(-7) M) produced an additive effect on TRH-induced PRL release. Pretreatment of anterior pituitary cells with pertussis toxin (30 ng/ml, 24 h) inhibited the LY-stimulated increase in plaque area. These findings indicate that very low concentrations of DA agonist stimulate the secretion of PRL per cell, and that the stimulatory effects of DA agonist on PRL secretion may be mediated by a pertussis toxin-sensitive G protein.
Stimulatory effects of quinpirole hydrochloride, D2-dopamine receptor agonist, at low concentrations on prolactin release in female rats in vitro.[Pubmed:1355255]
Life Sci. 1992;51(10):727-32.
Dopamine (DA) has dual actions (inhibitory and stimulatory) in the regulation of prolactin (PRL) release, depending on its concentration. To investigate the stimulatory effects of DA, perifused rat anterior pituitary cells were exposed to the highly-specific DA D2 receptor agonist, quinpirole hydrochloride (LY). Very low concentrations of LY (10(-12)-10(-10) M) stimulated PRL release and potentiated thyrotropin-releasing hormone (TRH)-induced PRL release. Higher concentrations of LY did not stimulate. Pretreatment with pertussis toxin (30 ng/ml, 24 h) completely abolished these effects of LY. The D2 receptor antagonist, metoclopramide, also blocked the potentiation by LY of TRH-induced PRL release. These data indicate that very low concentrations of dopamine stimulate PRL release via an interaction with a D2 receptor connected to a pertussis toxin-sensitive G protein.
Effects of quinpirole on central dopamine systems in sensitized and non-sensitized rats.[Pubmed:9483561]
Neuroscience. 1998 Apr;83(3):781-9.
The present study examined post mortem changes in central dopaminergic terminal regions following acute or chronic treatment regimens with the dopamine D2/D3 receptor agonist quinpirole, a psychomotor stimulant which induces pronounced behavioural sensitization when given chronically. Drug-induced changes in nucleus accumbens, striatum and amygdala were bilateral in nature, while in prefrontal cortex (medial prefrontal and anterior cingulate combined), left and right brain regions responded differentially to quinpirole. Acute drug treatment increased dopamine tissue levels in nucleus accumbens and right prefrontal cortex, while the dopamine metabolite 3,4-dihydroxyphenylacetic acid, was decreased in amygdala. In contrast, sensitization to quinpirole was associated with decreased dopamine levels in left prefrontal cortex, and increases in 3,4-dihydroxyphenylacetic acid levels in subcortical structures, particularly striatum and amygdala. Additionally, the increase in striatal 3,4-dihydroxyphenylacetic acid in chronic quinpirole animals was independent of drug treatment on the final day of injections. In summary, quinpirole induces a variety of simultaneous, regional changes in dopaminergic function, with the sensitized condition being primarily associated with an up-regulation of subcortical dopamine activity. While the nucleus accumbens and striatum play a well known role in motor activation and sensitized behaviour, it is concluded that the amygdala and prefrontal cortex have significant modulatory influences on these processes, with the role of the prefrontal cortex being asymmetrical in nature. Given the suggested relevance of behavioural sensitization to psychopathological states in humans, parallels are drawn between the present data and clinical findings, particularly in relation to obsessive-compulsive disorder.
Modulation of [3H]quinpirole binding in brain by monoamine oxidase inhibitors: evidence for a potential novel binding site.[Pubmed:8764345]
J Pharmacol Exp Ther. 1996 Jul;278(1):145-53.
[3H]Quinpirole is a dopamine agonist with high affinity for the D2 and D3 dopamine receptor subtypes. A variety of drugs, most notably monoamine oxidase inhibitors (MAOls), inhibit the binding of [3H]quinpirole, but not [3H]spiperone or [3H](-)N-n-Propylnorapomorphine, in rat striatal membranes by a mechanism that does not appear to involve the enzymatic activity of MAO. This study extends the characterization of MAOI-displaceable [3H]quinpirole binding in rat brain. Clinically antidepressant MAOIs exhibited selectivity between sites labeled by [3H]quinpirole and [3H]spiperone as did a number of structurally related propargylamines and N-acylethylenediamine derivatives and other drugs such as debrisoquin and phenylbiguanide. The MAOIs clorgyline and Ro 41-1049 were the most potent. Anti-depressant MAOIs inhibited [3H]quinpirole binding with the following rank order of potency: phenelzine > pargyline > tranyl-cypromine > isocarboxazid > nialamide > moclobemide. In striatal membranes, MAOI Ro 41-1049 inhibited [3H]quinpirole binding with similar potency at a variety of incubation temperatures (4-37 degrees C), assay tissue concentrations (5-20 mg original wet weight/ml), and time points (2 min-4 hr) and in the presence or absence of K+, Mg2+, Ca2+ ions, ascorbate, EDTA and NaCl. The regional distribution of Ro 41-1049-displaceable [3H]quinpirole binding in brain paralleled that of D2-like receptors. These data suggest that MAOIs interact with a novel binding site that is labeled by [3H]quinpirole or that modulates [3H]quinpirole binding. This site may be associated with D2-like dopamine receptors.
Dopamine receptor pharmacology.[Pubmed:7940991]
Trends Pharmacol Sci. 1994 Jul;15(7):264-70.
Dopamine receptors are the primary targets in the treatment of schizophrenia, Parkinson's disease, and Huntington's chorea, and are discussed in this review by Philip Seeman and Hubert Van Tol. Improved therapy may be obtained by drugs that selectively target a particular subtype of dopamine receptor. Most antipsychotic drugs block D2 receptors in direct correlation to clinical potency, except clozapine, which prefers D4 receptors. D1 and D2 receptors can enhance each other's actions, possibly through subunits of the G proteins. In schizophrenia, the D2 and D3 receptor density is elevated by 10%, while the D4 receptor density is elevated by 600%. Therefore, D4 receptors may be a target for future antipsychotic drugs. While antipsychotics originally helped to discover dopamine receptors, the five cloned dopamine receptors are now facilitating the discovery of selective antipsychotic and antiparkinson drugs.


