HA 130Selective autotaxin inhibitor CAS# 1229652-21-4 |
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1229652-21-4 | SDF | Download SDF |
| PubChem ID | 46911532 | Appearance | Powder |
| Formula | C24H19BFNO5S | M.Wt | 463.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 39 mg/mL (84.18 mM) *"≥" means soluble, but saturation unknown. | ||
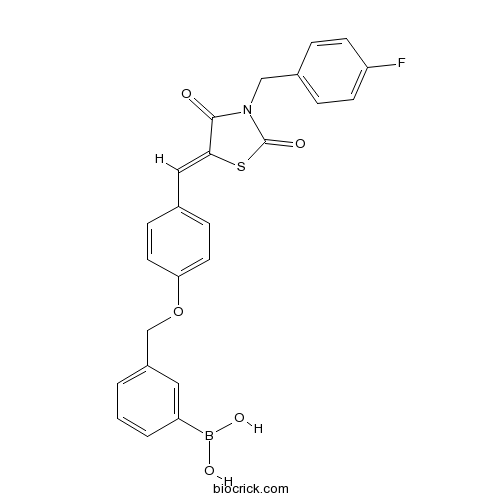
| Chemical Name | [3-[[4-[(Z)-[3-[(4-fluorophenyl)methyl]-2,4-dioxo-1,3-thiazolidin-5-ylidene]methyl]phenoxy]methyl]phenyl]boronic acid | ||
| SMILES | B(C1=CC(=CC=C1)COC2=CC=C(C=C2)C=C3C(=O)N(C(=O)S3)CC4=CC=C(C=C4)F)(O)O | ||
| Standard InChIKey | VTNKMYWFWQTEHE-XKZIYDEJSA-N | ||
| Standard InChI | InChI=1S/C24H19BFNO5S/c26-20-8-4-17(5-9-20)14-27-23(28)22(33-24(27)29)13-16-6-10-21(11-7-16)32-15-18-2-1-3-19(12-18)25(30)31/h1-13,30-31H,14-15H2/b22-13- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective autotaxin inhibitor (IC50 = 28 nM). Binds and inhibits autotaxin reversibly. Displays no activity against the proteasome, recombinant NPP1 or alkaline phosphatase. Inhibits ATX-mediated cell migration in an A2058 melanoma cell assay. |

HA 130 Dilution Calculator

HA 130 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1585 mL | 10.7924 mL | 21.5848 mL | 43.1695 mL | 53.9619 mL |
| 5 mM | 0.4317 mL | 2.1585 mL | 4.317 mL | 8.6339 mL | 10.7924 mL |
| 10 mM | 0.2158 mL | 1.0792 mL | 2.1585 mL | 4.317 mL | 5.3962 mL |
| 50 mM | 0.0432 mL | 0.2158 mL | 0.4317 mL | 0.8634 mL | 1.0792 mL |
| 100 mM | 0.0216 mL | 0.1079 mL | 0.2158 mL | 0.4317 mL | 0.5396 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6'-O-Galloyl paeoniflorin
Catalog No.:BCN2941
CAS No.:122965-41-7
- URMC-099
Catalog No.:BCC5563
CAS No.:1229582-33-5
- XE 991 dihydrochloride
Catalog No.:BCC7232
CAS No.:122955-13-9
- N-(3-Methoxybenzyl)palmitamide
Catalog No.:BCN8086
CAS No.:847361-96-0
- Fmoc-L-Beta-Homoproline
Catalog No.:BCN8087
CAS No.:193693-60-6
- LY2784544
Catalog No.:BCC2200
CAS No.:1229236-86-5
- C 21
Catalog No.:BCC8013
CAS No.:1229236-78-5
- GS-9973
Catalog No.:BCC5278
CAS No.:1229208-44-9
- Edoxaban tosylate monohydrate
Catalog No.:BCC1545
CAS No.:1229194-11-9
- 3-O-Acetylandrostenone hydrazone
Catalog No.:BCC8637
CAS No.:122914-94-7
- GSK 9027
Catalog No.:BCC6115
CAS No.:1229096-88-1
- Tatarinoid A
Catalog No.:BCN6119
CAS No.:1229005-35-9
- RG7388
Catalog No.:BCC1895
CAS No.:1229705-06-9
- Fmoc-Hyp(tBu)-OH
Catalog No.:BCC3256
CAS No.:122996-47-8
- 4-Hydroxybenzaldehyde
Catalog No.:BCN5816
CAS No.:123-08-0
- Anisic aldehyde
Catalog No.:BCN2618
CAS No.:123-11-5
- D-erythro-Sphingosine (synthetic)
Catalog No.:BCC6729
CAS No.:123-78-4
- Azelaic Acid
Catalog No.:BCC8300
CAS No.:123-99-9
- Azasetron HCl
Catalog No.:BCC5035
CAS No.:123040-16-4
- Bulleyanin
Catalog No.:BCN6120
CAS No.:123043-54-9
- Phyltetralin
Catalog No.:BCN3051
CAS No.:123048-17-9
- BAF312 (Siponimod)
Catalog No.:BCC5114
CAS No.:1230487-00-9
- 4,8-Dihydroxyeudesm-7(11)-en-12,8-olide
Catalog No.:BCN1600
CAS No.:1231208-53-9
- Uncaric acid
Catalog No.:BCN6121
CAS No.:123135-05-7
Discovery and optimization of boronic acid based inhibitors of autotaxin.[Pubmed:20536182]
J Med Chem. 2010 Jul 8;53(13):4958-67.
Autotaxin (ATX) is an extracellular enzyme that hydrolyzes lysophosphatidylcholine (LPC) to produce the lipid mediator lysophosphatidic acid (LPA). The ATX-LPA signaling axis has been implicated in diverse physiological and pathological processes, including vascular development, inflammation, fibrotic disease, and tumor progression. Therefore, targeting ATX with small molecule inhibitors is an attractive therapeutic strategy. We recently reported that 2,4-thiazolidinediones inhibit ATX activity in the micromolar range. Interestingly, inhibitory potency was dramatically increased by introduction of a boronic acid moiety, designed to target the active site threonine in ATX. Here we report on the discovery and further optimization of boronic acid based ATX inhibitors. The most potent of these compounds inhibits ATX-mediated LPC hydrolysis in the nanomolar range (IC(50) = 6 nM). The finding that ATX can be targeted by boronic acids may aid the development of ATX inhibitors for therapeutic use.
Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation.[Pubmed:20360563]
Proc Natl Acad Sci U S A. 2010 Apr 20;107(16):7257-62.
Autotaxin (ATX) is a secreted nucleotide pyrophosphatase/phosphodiesterase that functions as a lysophospholipase D to produce the lipid mediator lysophosphatidic acid (LPA), a mitogen, chemoattractant, and survival factor for many cell types. The ATX-LPA signaling axis has been implicated in angiogenesis, chronic inflammation, fibrotic diseases and tumor progression, making this system an attractive target for therapy. However, potent and selective nonlipid inhibitors of ATX are currently not available. By screening a chemical library, we have identified thiazolidinediones that selectively inhibit ATX-mediated LPA production both in vitro and in vivo. Inhibitor potency was approximately 100-fold increased (IC(50) approximately 30 nM) after the incorporation of a boronic acid moiety, designed to target the active-site threonine (T210) in ATX. Intravenous injection of this inhibitor into mice resulted in a surprisingly rapid decrease in plasma LPA levels, indicating that turnover of LPA in the circulation is much more dynamic than previously appreciated. Thus, boronic acid-based small molecules hold promise as candidate drugs to target ATX.


