D-erythro-Sphingosine (synthetic)Protein kinase C inhibitor CAS# 123-78-4 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Monomethyl auristatin E
Catalog No.:BCC1775
CAS No.:474645-27-7
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 123-78-4 | SDF | Download SDF |
| PubChem ID | 5280335 | Appearance | Powder |
| Formula | C18H37NO2 | M.Wt | 299.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 41.67 mg/mL (139.14 mM; Need ultrasonic) | ||
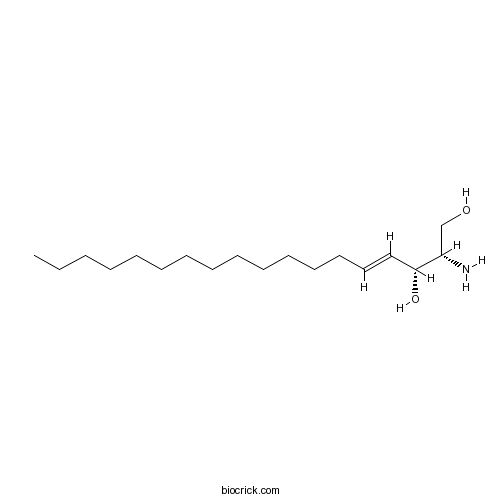
| Chemical Name | (E,2S,3R)-2-aminooctadec-4-ene-1,3-diol | ||
| SMILES | CCCCCCCCCCCCCC=CC(C(CO)N)O | ||
| Standard InChIKey | WWUZIQQURGPMPG-KRWOKUGFSA-N | ||
| Standard InChI | InChI=1S/C18H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(21)17(19)16-20/h14-15,17-18,20-21H,2-13,16,19H2,1H3/b15-14+/t17-,18+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of protein kinase C and calmodulin-dependent enzymes, but may stimulate mast cells by activation of protein kinase C. |

D-erythro-Sphingosine (synthetic) Dilution Calculator

D-erythro-Sphingosine (synthetic) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3389 mL | 16.6945 mL | 33.389 mL | 66.778 mL | 83.4725 mL |
| 5 mM | 0.6678 mL | 3.3389 mL | 6.6778 mL | 13.3556 mL | 16.6945 mL |
| 10 mM | 0.3339 mL | 1.6694 mL | 3.3389 mL | 6.6778 mL | 8.3472 mL |
| 50 mM | 0.0668 mL | 0.3339 mL | 0.6678 mL | 1.3356 mL | 1.6694 mL |
| 100 mM | 0.0334 mL | 0.1669 mL | 0.3339 mL | 0.6678 mL | 0.8347 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Anisic aldehyde
Catalog No.:BCN2618
CAS No.:123-11-5
- 4-Hydroxybenzaldehyde
Catalog No.:BCN5816
CAS No.:123-08-0
- Fmoc-Hyp(tBu)-OH
Catalog No.:BCC3256
CAS No.:122996-47-8
- RG7388
Catalog No.:BCC1895
CAS No.:1229705-06-9
- HA 130
Catalog No.:BCC7884
CAS No.:1229652-21-4
- 6'-O-Galloyl paeoniflorin
Catalog No.:BCN2941
CAS No.:122965-41-7
- URMC-099
Catalog No.:BCC5563
CAS No.:1229582-33-5
- XE 991 dihydrochloride
Catalog No.:BCC7232
CAS No.:122955-13-9
- N-(3-Methoxybenzyl)palmitamide
Catalog No.:BCN8086
CAS No.:847361-96-0
- Fmoc-L-Beta-Homoproline
Catalog No.:BCN8087
CAS No.:193693-60-6
- LY2784544
Catalog No.:BCC2200
CAS No.:1229236-86-5
- C 21
Catalog No.:BCC8013
CAS No.:1229236-78-5
- Azelaic Acid
Catalog No.:BCC8300
CAS No.:123-99-9
- Azasetron HCl
Catalog No.:BCC5035
CAS No.:123040-16-4
- Bulleyanin
Catalog No.:BCN6120
CAS No.:123043-54-9
- Phyltetralin
Catalog No.:BCN3051
CAS No.:123048-17-9
- BAF312 (Siponimod)
Catalog No.:BCC5114
CAS No.:1230487-00-9
- 4,8-Dihydroxyeudesm-7(11)-en-12,8-olide
Catalog No.:BCN1600
CAS No.:1231208-53-9
- Uncaric acid
Catalog No.:BCN6121
CAS No.:123135-05-7
- Cefepime Dihydrochloride Monohydrate
Catalog No.:BCC5261
CAS No.:123171-59-5
- LY2835219 free base
Catalog No.:BCC1722
CAS No.:1231929-97-7
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- VE-821
Catalog No.:BCC1207
CAS No.:1232410-49-9
- 6alpha-Hydroxytomentosin
Catalog No.:BCN7303
CAS No.:1232676-22-0
Sphingosine inhibition and promotion of histamine release from isolated rat mast cells.[Pubmed:1707581]
Agents Actions. 1990 Nov;31(3-4):171-67.
Sphingosine inhibited the histamine release induced by antigen, compound 48/80 with and without calcium, and the combination of TPA and the ionophore A23187. The inhibition occurred in the concentration range 1-3 microM, where no sign of cytotoxicity was noted. Preincubation for 5-10 min was needed for inhibition, and the effect persisted after washing of the cells. No inhibition was found with optimal concentrations of the ionophore or with TPA present during the preincubation. Sphingosine in combination with suboptimal concentrations of the ionophore could induce a considerable histamine release. This response was dependent on energy and was potently inhibited by the flavonoid phloretin. After preincubation with TPA, sphingosine exerted a pronounced potentiation of the response to very low concentrations of the ionophore. The findings regarding inhibitory effects of sphingosine do not seem to be compatible with a selective action on protein kinase C. The ability to synergize with the ionophore and to potentiate the effect of preincubation with TPA resembles previous findings with palmitoylcarnitine and suggests that sphingosine can stimulate mast cells by activation of protein kinase C.
Structural requirements for long-chain (sphingoid) base inhibition of protein kinase C in vitro and for the cellular effects of these compounds.[Pubmed:2742830]
Biochemistry. 1989 Apr 18;28(8):3138-45.
Sphingosine, sphinganine, and other long-chain (sphingoid) bases inhibit protein kinase C in vitro and block cellular responses to agonists that are thought to act via this enzyme. To gain further insight into the mechanism of this inhibition, a series of long-chain analogues differing in alkyl chain length (11-20 carbon atoms), stereochemistry, and headgroup were examined for (a) inhibition of protein kinase C activity in vitro, (b) the neutrophil respiratory burst in response to phorbol myristate acetate (PMA), (c) the PMA-induced differentiation of HL-60 cells, and (d) the growth of Chinese hamster ovary cells. In every instance, the effects were maximal with the 18-carbon homologues, which are the same length as the predominant naturally occurring long-chain base (sphingosine). The lower potency of the shorter chain homologues was partially due to decreased uptake by cells. Small differences were obtained with the four stereoisomers of sphingosine (i.e., D and L forms of erythro- and threo-sphingosine), with N-methyl derivatives of the different sphingosine homologues, and with simpler alkylamines (e.g., stearylamine). The potency of the different headgroup analogues may be affected by the degree of protonation at the assay pH. The pKa of sphingosine was measured to be 6.7; the pKa varied among the analogues. These findings establish that the major structural features required for inhibition of protein kinase C and cellular processes dependent on this enzyme are the presence of a free amino group and an aliphatic side chain and that other groups have more subtle effects.(ABSTRACT TRUNCATED AT 250 WORDS)


