RG7388MDM2 antagonist, oral, selective CAS# 1229705-06-9 |
- RG7388
Catalog No.:BCC1895
CAS No.:1229705-06-9
- MI-773
Catalog No.:BCC5155
CAS No.:1303607-07-9
- Nutlin-3a chiral
Catalog No.:BCC1812
CAS No.:675576-98-4
- MIRA-1
Catalog No.:BCC2409
CAS No.:72835-26-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1229705-06-9 | SDF | Download SDF |
| PubChem ID | 53358942 | Appearance | Powder |
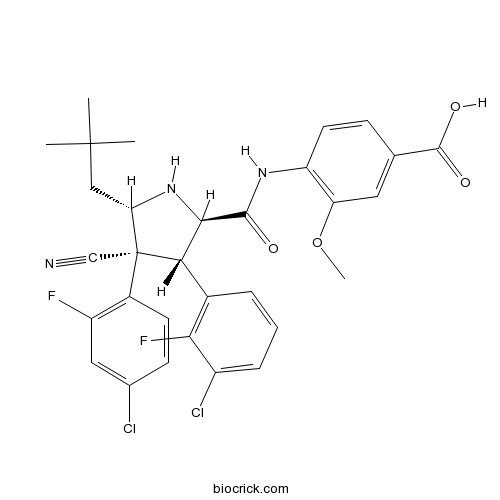
| Formula | C31H29Cl2F2N3O4 | M.Wt | 616.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Idasanutlin | ||
| Solubility | DMSO : ≥ 45 mg/mL (73.00 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-[[(2R,3S,4R,5S)-3-(3-chloro-2-fluorophenyl)-4-(4-chloro-2-fluorophenyl)-4-cyano-5-(2,2-dimethylpropyl)pyrrolidine-2-carbonyl]amino]-3-methoxybenzoic acid | ||
| SMILES | CC(C)(C)CC1C(C(C(N1)C(=O)NC2=C(C=C(C=C2)C(=O)O)OC)C3=C(C(=CC=C3)Cl)F)(C#N)C4=C(C=C(C=C4)Cl)F | ||
| Standard InChIKey | TVTXCJFHQKSQQM-LJQIRTBHSA-N | ||
| Standard InChI | InChI=1S/C31H29Cl2F2N3O4/c1-30(2,3)14-24-31(15-36,19-10-9-17(32)13-21(19)34)25(18-6-5-7-20(33)26(18)35)27(38-24)28(39)37-22-11-8-16(29(40)41)12-23(22)42-4/h5-13,24-25,27,38H,14H2,1-4H3,(H,37,39)(H,40,41)/t24-,25-,27+,31-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | RG7388 is a potent and selective MDM2 antagonist, inhibiting p53-MDM2 binding, with IC50 of 6 nM.In Vitro:RG7388 (Idasanutlin) inhibits cell proliferation with IC50 of 30 nM, and induces dose-dependent p53 stabilization, cell cycle arrest, as well as cell apoptosis in cancer cells expressing wild-type p53[1]. RG7388 (300 nM or 1.8 μM) induces apoptosis in SJSA osteosarcoma cells[2].In Vivo:RG7388 (Idasanutlin, 25 mg/kg p.o.) results in tumor growth inhibition and regression, in the mouse SJSA human osteosarcoma xenograft model[1]. RG7388 induces induction of apoptosis and antiproliferation, in the SJSA xenograft model[2]. References: | |||||

RG7388 Dilution Calculator

RG7388 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6221 mL | 8.1106 mL | 16.2211 mL | 32.4423 mL | 40.5528 mL |
| 5 mM | 0.3244 mL | 1.6221 mL | 3.2442 mL | 6.4885 mL | 8.1106 mL |
| 10 mM | 0.1622 mL | 0.8111 mL | 1.6221 mL | 3.2442 mL | 4.0553 mL |
| 50 mM | 0.0324 mL | 0.1622 mL | 0.3244 mL | 0.6488 mL | 0.8111 mL |
| 100 mM | 0.0162 mL | 0.0811 mL | 0.1622 mL | 0.3244 mL | 0.4055 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
RG7388 is a second generation clinical MDM2 inhibitor with superior potency and selectivity.
It is a highly potent pyrrolidine compound. RG7388 is more potent and selective than RG7112. In human cancer cell lines, IC50 value of RG7388 in HTRF binding assays is 6 nM and IC50 value of RG7388 in MTT proliferation assays is 0.03μM. [1]
In human cancer cell lines, RG7388 blocks p53−MDM2 binding and effectively activates the p53 pathway, leading to cell cycle arrest and/or apoptosis in cell lines expressing wild-type p53 and tumor growth inhibition or regression of osteosarcoma xenografts in nude mice. RG7388 is undergoing clinical investigation in solid and hematological tumors. [1]
In rhabdomyosarcoma xenografts mice, RG7388 increased the activity of Ionizing radiation (XRT) in both rhabdomyosarcoma models and did not increasing local XRT-induced skin toxicity. Changes in TP53-responsive genes were consistent with the synergistic activity of RG7388 and XRT in the Rh18 model. [2]
RG7388 GI50 concentrations of wt p53 was a >200-fold difference versus mutant cell lines. Comparing with MYCN- cells, Tet21N MYCN+ cells were more sensitive to RG7388. In five p53-wt neuroblastoma cell lines, combining use of RG7388 with cisplatin, topotecan, doxorubicin, busulfan and temozolomide were synergistic led to increased apoptosis and higher caspase-3/7 activity. RG7388 is highly potent against p53-wt neuroblastoma cells, and strongly supports its further evaluation as a novel therapy for patients with high-risk neuroblastoma and wt p53 to potentially improve survival and/or reduce toxicity. [3]
References:
1.Ding Q, Zhang Z, Liu JJ et al. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J Med Chem. 2013 Jul 25;56(14):5979-83.
2.Phelps D, Bondra K, Seum S et al. Inhibition of MDM2 by RG7388 confers hypersensitivity to X-radiation in xenograft models of childhood sarcoma. Pediatr Blood Cancer. 2015 Apr 1. doi: 10.1002/pbc.25465.
3.Chen L, Rousseau RF, Middleton SA et al. Pre-clinical evaluation of the MDM2-p53 antagonist RG7388 alone and in combination with chemotherapy in neuroblastoma. Oncotarget. 2015 Apr 30;6(12):10207-21.
- HA 130
Catalog No.:BCC7884
CAS No.:1229652-21-4
- 6'-O-Galloyl paeoniflorin
Catalog No.:BCN2941
CAS No.:122965-41-7
- URMC-099
Catalog No.:BCC5563
CAS No.:1229582-33-5
- XE 991 dihydrochloride
Catalog No.:BCC7232
CAS No.:122955-13-9
- N-(3-Methoxybenzyl)palmitamide
Catalog No.:BCN8086
CAS No.:847361-96-0
- Fmoc-L-Beta-Homoproline
Catalog No.:BCN8087
CAS No.:193693-60-6
- LY2784544
Catalog No.:BCC2200
CAS No.:1229236-86-5
- C 21
Catalog No.:BCC8013
CAS No.:1229236-78-5
- GS-9973
Catalog No.:BCC5278
CAS No.:1229208-44-9
- Edoxaban tosylate monohydrate
Catalog No.:BCC1545
CAS No.:1229194-11-9
- 3-O-Acetylandrostenone hydrazone
Catalog No.:BCC8637
CAS No.:122914-94-7
- GSK 9027
Catalog No.:BCC6115
CAS No.:1229096-88-1
- Fmoc-Hyp(tBu)-OH
Catalog No.:BCC3256
CAS No.:122996-47-8
- 4-Hydroxybenzaldehyde
Catalog No.:BCN5816
CAS No.:123-08-0
- Anisic aldehyde
Catalog No.:BCN2618
CAS No.:123-11-5
- D-erythro-Sphingosine (synthetic)
Catalog No.:BCC6729
CAS No.:123-78-4
- Azelaic Acid
Catalog No.:BCC8300
CAS No.:123-99-9
- Azasetron HCl
Catalog No.:BCC5035
CAS No.:123040-16-4
- Bulleyanin
Catalog No.:BCN6120
CAS No.:123043-54-9
- Phyltetralin
Catalog No.:BCN3051
CAS No.:123048-17-9
- BAF312 (Siponimod)
Catalog No.:BCC5114
CAS No.:1230487-00-9
- 4,8-Dihydroxyeudesm-7(11)-en-12,8-olide
Catalog No.:BCN1600
CAS No.:1231208-53-9
- Uncaric acid
Catalog No.:BCN6121
CAS No.:123135-05-7
- Cefepime Dihydrochloride Monohydrate
Catalog No.:BCC5261
CAS No.:123171-59-5
Antitumour activity of the glycoengineered type II anti-CD20 antibody obinutuzumab (GA101) in combination with the MDM2-selective antagonist idasanutlin (RG7388).[Pubmed:26993060]
Eur J Haematol. 2016 Nov;97(5):461-470.
OBJECTIVES: To investigate whether the glycoengineered type II anti-CD20 monoclonal antibody obinutuzumab (GA101) combined with the selective MDM2 antagonist idasanutlin (RG7388) offers superior efficacy to monotherapy in treating B-lymphoid malignancies in preclinical models. METHODS: The combined effect of obinutuzumab or rituximab plus idasanutlin on direct cell death/apoptosis induction and antibody-dependent cellular cytotoxicity (ADCC) was evaluated using p53 wild-type Z-138 and DoHH-2 lymphoma cells. Furthermore, whole blood B-cell depletion was analysed, and tumour growth inhibition was evaluated in subcutaneous xenograft models. RESULTS: Idasanutlin induced concentration-dependent death of Z-138 and DoHH-2 cells. At concentrations >10-100 nm, idasanutlin enhanced obinutuzumab-induced death of DoHH-2 and Z-138 cells without negatively impacting obinutuzumab-mediated ADCC, natural killer cell activation or whole blood B-cell depletion. In the Z-138 xenograft model, a suboptimal dose of obinutuzumab with idasanutlin yielded substantial tumour growth inhibition and prolonged survival in a time-to-event analysis. In the DoHH-2 model, idasanutlin plus obinutuzumab showed superior tumour growth inhibition to idasanutlin plus rituximab. CONCLUSIONS: Obinutuzumab plus idasanutlin enhanced cell death of p53 wild-type tumour cells vs. rituximab plus idasanutlin without affecting obinutuzumab-mediated ADCC or B-cell depletion and showed robust antitumour efficacy in xenograft models, strongly supporting the investigation of this combination in clinical trials.
Pre-clinical efficacy and synergistic potential of the MDM2-p53 antagonists, Nutlin-3 and RG7388, as single agents and in combined treatment with cisplatin in ovarian cancer.[Pubmed:27223080]
Oncotarget. 2016 Jun 28;7(26):40115-40134.
Ovarian cancer is the fifth leading cause of cancer-related female deaths. Due to serious side effects, relapse and resistance to standard chemotherapy, better and more targeted approaches are required. Mutation of the TP53 gene accounts for 50% of all human cancers. In the remaining malignancies, non-genotoxic activation of wild-type p53 by small molecule inhibition of the MDM2-p53 binding interaction is a promising therapeutic strategy. Proof of concept was established with the cis-imidazoline Nutlin-3, leading to the development of RG7388 and other compounds currently in early phase clinical trials. This preclinical study evaluated the effect of Nutlin-3 and RG7388 as single agents and in combination with cisplatin in a panel of ovarian cancer cell lines. Median-drug-effect analysis showed Nutlin-3 or RG7388 combination with cisplatin was additive to, or synergistic in a p53-dependent manner, resulting in increased p53 activation, cell cycle arrest and apoptosis, associated with increased p21WAF1 protein and/or caspase-3/7 activity compared to cisplatin alone. Although MDM2 inhibition activated the expression of p53-dependent DNA repair genes, the growth inhibitory and pro-apoptotic effects of p53 dominated the response. These data indicate that combination treatment with MDM2 inhibitors and cisplatin has synergistic potential for the treatment of ovarian cancer, dependent on cell genotype.
Pre-clinical evaluation of the MDM2-p53 antagonist RG7388 alone and in combination with chemotherapy in neuroblastoma.[Pubmed:25844600]
Oncotarget. 2015 Apr 30;6(12):10207-21.
Neuroblastoma is a predominantly p53 wild-type (wt) tumour and MDM2-p53 antagonists offer a novel therapeutic strategy for neuroblastoma patients. RG7388 (Roche) is currently undergoing early phase clinical evaluation in adults. This study assessed the efficacy of RG7388 as a single-agent and in combination with chemotherapies currently used to treat neuroblastoma in a panel of neuroblastoma cell lines. RG7388 GI50 concentrations were determined in 21 p53-wt and mutant neuroblastoma cell lines of varying MYCN, MDM2 and p14(ARF) status, together with MYCN-regulatable Tet21N cells. The primary determinant of response was the presence of wt p53, and overall there was a >200-fold difference in RG7388 GI50 concentrations for p53-wt versus mutant cell lines. Tet21N MYCN+ cells were significantly more sensitive to RG7388 compared with MYCN- cells. Using median-effect analysis in 5 p53-wt neuroblastoma cell lines, selected combinations of RG7388 with cisplatin, doxorubicin, topotecan, temozolomide and busulfan were synergistic. Furthermore, combination treatments led to increased apoptosis, as evident by higher caspase-3/7 activity compared to either agent alone. These data show that RG7388 is highly potent against p53-wt neuroblastoma cells, and strongly supports its further evaluation as a novel therapy for patients with high-risk neuroblastoma and wt p53 to potentially improve survival and/or reduce toxicity.


