alpha-ConidendrinCAS# 85699-62-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 85699-62-3 | SDF | Download SDF |
| PubChem ID | 369613 | Appearance | Powder |
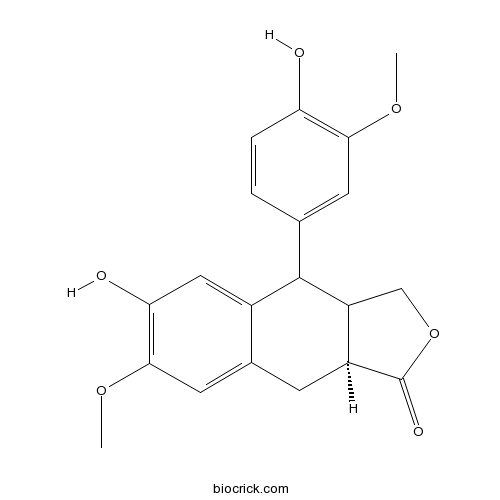
| Formula | C20H20O6 | M.Wt | 356.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3aS)-7-hydroxy-9-(4-hydroxy-3-methoxyphenyl)-6-methoxy-3a,4,9,9a-tetrahydro-1H-benzo[f][2]benzofuran-3-one | ||
| SMILES | COC1=C(C=C2C(C3COC(=O)C3CC2=C1)C4=CC(=C(C=C4)O)OC)O | ||
| Standard InChIKey | CAYMSCGTKZIVTN-CDJWAVJASA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Cytotoxic responses to aromatic ring and configurational variations in alpha-conidendrin, podophyllotoxin, and sikkimotoxin derivatives.[Pubmed: 11170627]J Med Chem. 2001 Jan 18;44(2):180-5.Derivatives of alpha-Conidendrin, podophyllotoxin, and sikkimotoxin were prepared to evaluate the cytotoxic contributions of C-4 configuration and pendant and fused arene substitutions.
|
| Structure Identification | J Nat Prod. 2004 Apr;67(4):697-9.Alpha-conidendrin as a source for preparation of sikkimotoxin derivatives.[Pubmed: 15104508]
|

alpha-Conidendrin Dilution Calculator

alpha-Conidendrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8058 mL | 14.0292 mL | 28.0584 mL | 56.1167 mL | 70.1459 mL |
| 5 mM | 0.5612 mL | 2.8058 mL | 5.6117 mL | 11.2233 mL | 14.0292 mL |
| 10 mM | 0.2806 mL | 1.4029 mL | 2.8058 mL | 5.6117 mL | 7.0146 mL |
| 50 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1223 mL | 1.4029 mL |
| 100 mM | 0.0281 mL | 0.1403 mL | 0.2806 mL | 0.5612 mL | 0.7015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-Blebbistatin
Catalog No.:BCC4375
CAS No.:856925-71-8
- Tedizolid
Catalog No.:BCC1990
CAS No.:856866-72-3
- AM 114
Catalog No.:BCC3589
CAS No.:856849-35-9
- CBiPES hydrochloride
Catalog No.:BCC7824
CAS No.:856702-40-4
- Choline Fenofibrate
Catalog No.:BCC1478
CAS No.:856676-23-8
- Setiptiline maleate
Catalog No.:BCC1946
CAS No.:85650-57-3
- Asenapine
Catalog No.:BCC2476
CAS No.:85650-56-2
- Mirtazapine
Catalog No.:BCC4923
CAS No.:85650-52-8
- Laurycolactone B
Catalog No.:BCN3110
CAS No.:85643-77-2
- Laurycolactone A
Catalog No.:BCN3109
CAS No.:85643-76-1
- Curculigoside
Catalog No.:BCN4406
CAS No.:85643-19-2
- Boc-Ala-NH2
Catalog No.:BCC3046
CAS No.:85642-13-3
- (3S,3'R,8R,9R,9As)-8-methoxy-3'-methyl-3-[(2S,4S)-4-methyl-5-oxooxolan-2-yl]spiro[1,2,3,5,6,7,8,9a-octahydropyrrolo[1,2-a]azepine-9,5'-oxolane]-2'-one
Catalog No.:BCC9250
CAS No.:85700-47-6
- Scopine HCl
Catalog No.:BCC4940
CAS No.:85700-55-6
- WP1066
Catalog No.:BCC2194
CAS No.:857064-38-1
- TMC353121
Catalog No.:BCC2004
CAS No.:857066-90-1
- PF 915275
Catalog No.:BCC7631
CAS No.:857290-04-1
- 3-Hydroxysarpagine
Catalog No.:BCN4566
CAS No.:857297-90-6
- Isoflavidinin
Catalog No.:BCN7604
CAS No.:85734-02-7
- Retaspimycin
Catalog No.:BCC1889
CAS No.:857402-23-4
- IPI-504 (Retaspimycin hydrochloride)
Catalog No.:BCC2126
CAS No.:857402-63-2
- K-252c
Catalog No.:BCC3706
CAS No.:85753-43-1
- AT7867
Catalog No.:BCC2536
CAS No.:857531-00-1
- PSN632408
Catalog No.:BCC5408
CAS No.:857652-30-3
Cytotoxic responses to aromatic ring and configurational variations in alpha-conidendrin, podophyllotoxin, and sikkimotoxin derivatives.[Pubmed:11170627]
J Med Chem. 2001 Jan 18;44(2):180-5.
Derivatives of alpha-Conidendrin, podophyllotoxin, and sikkimotoxin were prepared to evaluate the cytotoxic contributions of C-4 configuration and pendant and fused arene substitutions. Dimethyl-alpha-conidendryl alcohol (5), 9-deoxypodophyllol (6), and 9-deoxysikkimol (17) were dehydrated to their respective oxolane derivatives 4, 3, and 9. Diols 5 and 6 were converted via oxabicyclo[3.2.1]octanols 10 and 14 to target oxolanes 8 and 7 where C-4 had been inverted relative to that in 3 and 4. Cytotoxicities of the five oxolanes were determined in two drug-sensitive human leukemia and two multidrug-resistant cell lines expressing P-glycoprotein or multidrug-resistance associated protein (MRP). Changing the pendant arene configuration or replacing a m-methoxy by hydrogen resulted in a 100-fold cytotoxicity loss. Replacing a methylenedioxy group in the fused arene by two methoxy substituents reduced cytotoxicity by 10-fold. Drug-resistant cell lines were equally resistant to compounds 3, 4, 8, and 9 indicating that these four compounds do not serve as substrates of the transport proteins P-glycoprotein and MRP.
Alpha-conidendrin as a source for preparation of sikkimotoxin derivatives.[Pubmed:15104508]
J Nat Prod. 2004 Apr;67(4):697-9.
Oxidation of alpha-Conidendrin (3) by Fremy's salt favored formation of an o-quinone (4) at the pendant aromatic ring as opposed to the fused aromatic ring. Quinone reduction and phenolic methylation, followed by lactone reduction, and subsequent oxidation by dichlorodicyanoquinone produced sikkimotoxin oxabicyclooctane (7), while oxidation with cupric sulfate/potassium persulfate gave sikkimotoxin dioxatricyclodecane (8).


