Palmatine hydrochlorideCAS# 10605-02-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10605-02-4 | SDF | Download SDF |
| PubChem ID | 73442 | Appearance | Yellow powder |
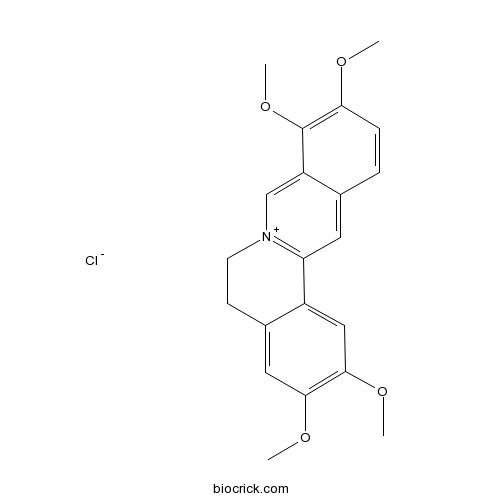
| Formula | C21H22ClNO4 | M.Wt | 387.86 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Fibrauretin chloride | ||
| Solubility | DMSO : ≥ 32 mg/mL (82.50 mM) H2O : 5 mg/mL (12.89 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2,3,9,10-tetramethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium;chloride | ||
| SMILES | COC1=C(C2=C[N+]3=C(C=C2C=C1)C4=CC(=C(C=C4CC3)OC)OC)OC.[Cl-] | ||
| Standard InChIKey | RLQYRXCUPVKSAW-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C21H22NO4.ClH/c1-23-18-6-5-13-9-17-15-11-20(25-3)19(24-2)10-14(15)7-8-22(17)12-16(13)21(18)26-4;/h5-6,9-12H,7-8H2,1-4H3;1H/q+1;/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Palmatine hydrochloride is a hydrochloride salt of palmatine which is a protoberberine alkaloid, it can induce remarkable cell apoptosis, has potential in photodynamic therapy on colon adenocarcinoma. Palmatine hydrochloride has anti-C. albicans effect, it mixes with berberine hydrochloride elicit antifungal activities, it also can reduce blood sugar and oxidative stress in STZ induced diabetic rats. |
| Targets | Antifection |
| In vitro | Investigation on the Anti-Candida albicans Effect of Palmatine Hydrochloride Based on Microcalorimetry and Principal Component Analysis[Reference: WebLink]Acta Chim. Sin. Chinese Edition, 2009, 67(21):2511-6.
|
| In vivo | Palmatine hydrochloride improves motor dysfunction in streptozotocin-induced diabetic rats.[Reference: WebLink]Proceedings of the National Academy of Sciences, 2014, 48(3):461-471.Diabetes induces motor dysfunctions, Palmatine is an isoquinoline alkaloid, with anti-diabetic and antioxidant activities. This study was conducted to evaluate the effect of Palmatine on motor dysfunction in STZ-induced diabetic rats.
|
| Cell Research | Photodynamic action of palmatine hydrochloride on colon adenocarcinoma HT-29 cells.[Pubmed: 27181460 ]Photodiagnosis Photodyn Ther. 2016 Sep;15:53-8.Palmatine hydrochloride (PaH) is a natural active compound from a traditional Chinese medicine (TCM). The present study aims to evaluate the effect of PaH as a new photosensitizer on colon adenocarcinoma HT-29 cells upon light irradiation.
|
| Structure Identification | Luminescence. 2014 May;29(3):211-8.Studies on the interaction of palmatine hydrochloride with bovine hemoglobin.[Pubmed: 23696111 ]The interaction between bovine hemoglobin (BHb) and Palmatine hydrochloride (PMT) was investigated at different temperatures using multispectroscopy, as well as the effect of common metal ions (Ca(2+) , Mg(2+) , Zn(2+) , Cu(2+) , Fe(2+) , Fe(3+) , Co(2+) , Ni(2+) ) on the BHb-PMT system. Results showed that the quenching mechanism of PMT on BHb was a static process. The electrostatic force played an important role in the conjugation reaction between BHb and PMT. The order of magnitude of the binding constants (Ka ) was 10(4) , and the number of binding sites (n) in the binary system was ~ 1. The binding distance (r) was ~ 2.44 nm and the primary binding for PMT was located at β-37 tryptophan in the hydrophobic cavity of BHb. In addition, the Hill's coefficients were ~ 1. Synchronous and circular dichroism spectra revealed that the microenvironment and the conformation of BHb were changed during the binding reaction. |

Palmatine hydrochloride Dilution Calculator

Palmatine hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5782 mL | 12.8912 mL | 25.7825 mL | 51.565 mL | 64.4562 mL |
| 5 mM | 0.5156 mL | 2.5782 mL | 5.1565 mL | 10.313 mL | 12.8912 mL |
| 10 mM | 0.2578 mL | 1.2891 mL | 2.5782 mL | 5.1565 mL | 6.4456 mL |
| 50 mM | 0.0516 mL | 0.2578 mL | 0.5156 mL | 1.0313 mL | 1.2891 mL |
| 100 mM | 0.0258 mL | 0.1289 mL | 0.2578 mL | 0.5156 mL | 0.6446 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Palmatine chloride an isoquinoline alkaloid, is an important medicinal herbal extract with diverse pharmacological and biological properties. IC50 value: Target: In vitro: Experimental set examined the influence of palmatine on osteoblast-like cells in vitro. In the culture supernatant of MC3T3-E1 cells, RANKL and OPG levels were significantly reduced by palmatine addition [1]. In vivo: The first experimentaI set was designed to histologically and biochemically examine mice randomly divided into four groups: sham-operated, OVX, and OVX-palmatine intake groups (1 mg/kg and 10 mg/kg). In palmatine-treated mice, RANKL and OPG expression decreased [1].
References:
[1]. SHINTARO ISHIKAWA, et al. Influence of Palmatine on Bone Metabolism in Ovariectomized Mice and Cytokine Secretion of Osteoblasts.
[2]. Chen-Wen Xiao, er al. Antifungal activity of berberine hydrochloride and palmatine hydrochloride against Microsporum canis -induced dermatitis in rabbits and underlying mechanism. BMC Complement Altern Med. 2015; 15: 177.
- β-Interleukin I (163-171), human
Catalog No.:BCC1017
CAS No.:106021-96-9
- Nerol
Catalog No.:BCN8517
CAS No.:106-25-2
- Geraniol
Catalog No.:BCN2631
CAS No.:106-24-1
- 2-(3,4-Dihydroxyphenyl)ethanol
Catalog No.:BCN5871
CAS No.:10597-60-1
- Sulfocostunolide B
Catalog No.:BCN5870
CAS No.:1059671-65-6
- Clinafloxacin CI96 AM1091
Catalog No.:BCC3754
CAS No.:105956-97-6
- OLDA
Catalog No.:BCC7138
CAS No.:105955-11-1
- STEARDA
Catalog No.:BCC7288
CAS No.:105955-10-0
- Doxycycline HCl
Catalog No.:BCC3772
CAS No.:10592-13-9
- Taraxasterol
Catalog No.:BCN5869
CAS No.:1059-14-9
- (tert-Butoxycarbonyl)oxycefcapene pivoxil
Catalog No.:BCC8403
CAS No.:105889-80-3
- Obtusilin
Catalog No.:BCN2697
CAS No.:105870-59-5
- Hoechst 33342 analog 2
Catalog No.:BCC1631
CAS No.:106050-84-4
- Senktide
Catalog No.:BCC6921
CAS No.:106128-89-6
- PND-1186
Catalog No.:BCC1866
CAS No.:1061353-68-1
- TC-G 1004
Catalog No.:BCC6165
CAS No.:1061747-72-5
- WAY-600
Catalog No.:BCC4607
CAS No.:1062159-35-6
- WYE-687
Catalog No.:BCC4604
CAS No.:1062161-90-3
- WYE-354
Catalog No.:BCC1059
CAS No.:1062169-56-5
- Ro3280
Catalog No.:BCC3962
CAS No.:1062243-51-9
- LDN-193189
Catalog No.:BCC3687
CAS No.:1062368-24-4
- ML347
Catalog No.:BCC5331
CAS No.:1062368-49-3
- LDN193189 Hydrochloride
Catalog No.:BCC1695
CAS No.:1062368-62-0
- Thioperamide
Catalog No.:BCC6734
CAS No.:106243-16-7
Photodynamic action of palmatine hydrochloride on colon adenocarcinoma HT-29 cells.[Pubmed:27181460]
Photodiagnosis Photodyn Ther. 2016 Sep;15:53-8.
Palmatine hydrochloride (PaH) is a natural active compound from a traditional Chinese medicine (TCM). The present study aims to evaluate the effect of PaH as a new photosensitizer on colon adenocarcinoma HT-29 cells upon light irradiation. Firstly, the absorption and fluorescence spectra of PaH were measured using a UV-vis spectrophotometer and RF-1500PC spectrophotometer, respectively. Singlet oxygen ((1)O2) production of PaH was determined using 1, 3-diphenylisobenzofuran (DPBF). Dark toxicity of PaH was estimated using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Cellular uptake of PaH in HT-29 cells was detected at different time intervals. Subellular localization of PaH in HT-29 cells was observed using confocal laser fluorescence microscopy. For photodynamic treatment, HT-29 cells were incubated with PaH and then irradiated by visible light (470nm) from a LED light source. Photocytotoxicity was investigated 24h after photodynamic treatment using MTT assay. Cell apoptosis was observed 18h after photodynamic treatment using a flow cytometry with Annexin V/PI staining. Results showed that PaH has an absorption peak in the visible region from 400nm to 500nm and a fluorescence emission peak at 406nm with an excitation wavelength of 365nm. PaH was activated by the 470nm visible light from a LED light source to produce (1)O2. Dark toxicity showed that PaH alone treatment had no cytotoxicity to HT-29 cancer cells and NIH-3T3 normal cells after incubation for 24h. After incubation for 40min, the cellular uptake of PaH reached to the maximum and PaH was located in mitochondria. Photodynamic treatment of PaH demonstrated a significant photocytotoxicity on HT-29 cells. The rate of cell death increased significantly in a PaH concentration-dependent and light dose-dependent manner. Further evaluation revealed that the early and late apoptotic rate of HT-29 cells increased remarkably up to 21.54% and 5.39% after photodynamic treatment of PaH at the concentration of 5muM and energy density of 10.8J/cm(2). Our findings demonstrated that PaH as a naturally occurring photosensitizer has potential in photodynamic therapy on colon adenocarcinoma.
Studies on the interaction of palmatine hydrochloride with bovine hemoglobin.[Pubmed:23696111]
Luminescence. 2014 May;29(3):211-8.
The interaction between bovine hemoglobin (BHb) and Palmatine hydrochloride (PMT) was investigated at different temperatures using multispectroscopy, as well as the effect of common metal ions (Ca(2+) , Mg(2+) , Zn(2+) , Cu(2+) , Fe(2+) , Fe(3+) , Co(2+) , Ni(2+) ) on the BHb-PMT system. Results showed that the quenching mechanism of PMT on BHb was a static process. The electrostatic force played an important role in the conjugation reaction between BHb and PMT. The order of magnitude of the binding constants (Ka ) was 10(4) , and the number of binding sites (n) in the binary system was ~ 1. The binding distance (r) was ~ 2.44 nm and the primary binding for PMT was located at beta-37 tryptophan in the hydrophobic cavity of BHb. In addition, the Hill's coefficients were ~ 1. Synchronous and circular dichroism spectra revealed that the microenvironment and the conformation of BHb were changed during the binding reaction.


