Hoechst 33258 analogCAS# 258843-62-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 258843-62-8 | SDF | Download SDF |
| PubChem ID | 12116202 | Appearance | Powder |
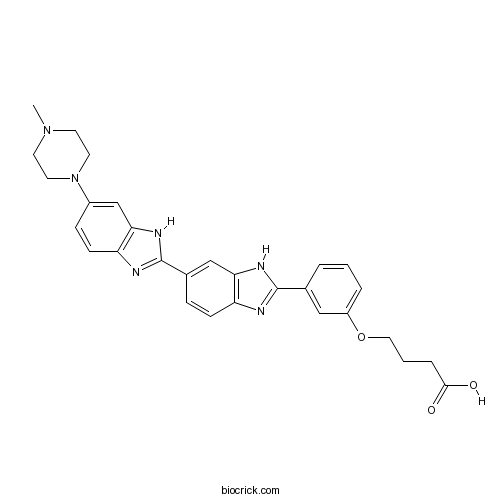
| Formula | C29H30N6O3 | M.Wt | 510.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO | ||
| Chemical Name | 4-[3-[6-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-1H-benzimidazol-2-yl]phenoxy]butanoic acid | ||
| SMILES | CN1CCN(CC1)C2=CC3=C(C=C2)N=C(N3)C4=CC5=C(C=C4)N=C(N5)C6=CC(=CC=C6)OCCCC(=O)O | ||
| Standard InChIKey | QXQZJBBLMSBZHP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H30N6O3/c1-34-11-13-35(14-12-34)21-8-10-24-26(18-21)33-29(31-24)20-7-9-23-25(17-20)32-28(30-23)19-4-2-5-22(16-19)38-15-3-6-27(36)37/h2,4-5,7-10,16-18H,3,6,11-15H2,1H3,(H,30,32)(H,31,33)(H,36,37) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Hoechst 33258 analog Dilution Calculator

Hoechst 33258 analog Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9585 mL | 9.7926 mL | 19.5852 mL | 39.1704 mL | 48.963 mL |
| 5 mM | 0.3917 mL | 1.9585 mL | 3.917 mL | 7.8341 mL | 9.7926 mL |
| 10 mM | 0.1959 mL | 0.9793 mL | 1.9585 mL | 3.917 mL | 4.8963 mL |
| 50 mM | 0.0392 mL | 0.1959 mL | 0.3917 mL | 0.7834 mL | 0.9793 mL |
| 100 mM | 0.0196 mL | 0.0979 mL | 0.1959 mL | 0.3917 mL | 0.4896 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Hoechst 33258 analog
- Liensinine
Catalog No.:BCN6337
CAS No.:2586-96-1
- H-D-Cys(Trt)-OH
Catalog No.:BCC2914
CAS No.:25840-82-8
- Ghrelin (rat)
Catalog No.:BCC5767
CAS No.:258338-12-4
- IEM 1925 dihydrobromide
Catalog No.:BCC7885
CAS No.:258282-23-4
- Ghrelin (human)
Catalog No.:BCC7076
CAS No.:258279-04-8
- LEP (116-130) (mouse)
Catalog No.:BCC1016
CAS No.:258276-95-8
- Gemfibrozil
Catalog No.:BCC4783
CAS No.:25812-30-0
- Pelitinib (EKB-569)
Catalog No.:BCC1118
CAS No.:257933-82-7
- H-D-Glu(OBzl)-OH
Catalog No.:BCC2939
CAS No.:2578-33-8
- CP 154526
Catalog No.:BCC7481
CAS No.:257639-98-8
- J 104129 fumarate
Catalog No.:BCC7389
CAS No.:257603-40-0
- Velutin
Catalog No.:BCN5130
CAS No.:25739-41-7
- Yunaconitoline
Catalog No.:BCN6703
CAS No.:259099-25-7
- D-Luciferin
Catalog No.:BCC6535
CAS No.:2591-17-5
- 3'-Hydroxydehydroaglaiastatin
Catalog No.:BCN7725
CAS No.:259143-58-3
- Boc-Thr-OH
Catalog No.:BCC3449
CAS No.:2592-18-9
- Boc-Lys(Boc)-ONp
Catalog No.:BCC3414
CAS No.:2592-19-0
- HOBt (anhydrous)
Catalog No.:BCC2816
CAS No.:2592-95-2
- EDC.HCl
Catalog No.:BCC2812
CAS No.:25952-53-8
- Allura Red AC
Catalog No.:BCN2220
CAS No.:25956-17-6
- 5-Acetyl-3-chloro-10,11-dihydro-5H-dibenz[b,f]azepine
Catalog No.:BCC8726
CAS No.:25961-11-9
- 5-Hydroxy-7-methoxy-3-(4-hydroxybenzylidene)chroman-4-one
Catalog No.:BCN1472
CAS No.:259653-54-8
- 2-Deacetyltaxuspine X
Catalog No.:BCN7375
CAS No.:259678-73-4
- T 705
Catalog No.:BCC4130
CAS No.:259793-96-9
Synthesis and sequence-specific DNA binding of a topoisomerase inhibitory analog of Hoechst 33258 designed for altered base and sequence recognition.[Pubmed:1280170]
Chem Res Toxicol. 1992 Sep-Oct;5(5):597-607.
The preparation and DNA binding characteristics of a structural analog of Hoechst 33258 bearing two pyridinic nitrogen atoms are described. The 1H NMR signals of the complex formed between the new ligand 1 and decadeoxyribonucleotide d(CATGGCCATG)2 were assigned by employing one- and two-dimensional NMR techniques. Intermolecular nuclear Overhauser effects (NOE) between the ligand and the DNA receptor fragment confirm that the ligand binds in the minor groove of the DNA, interacting with the centrally located 5'-GGCCA segment. In contrast to the steric clash between the benzimidazole rings of the parent Hoechst 33258 molecule and the guanine 2-NH2 groups, which renders it G.C avoiding and thus A.T base pair preferring, the ligand 1 described here overcomes these unfavorable interactions and instead exhibits a marked preference of G.C base pairs. This behavior appears to arise from additional stabilization due to H-bonding with the guanine 2-NH2 groups. Although a ligand-induced distortion at the binding site is qualitatively assessable, the overall B-type conformation of the DNA fragment is retained upon complexation. The structural conclusions drawn from the NOE-NMR evidence were confirmed by molecular mechanics and molecular modeling studies.


