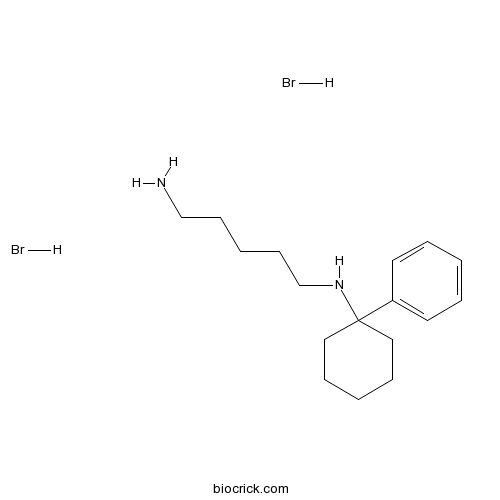
IEM 1925 dihydrobromideCAS# 258282-23-4 |
- NLG919
Catalog No.:BCC2325
CAS No.:1402836-58-1
- INCB024360 analogue
Catalog No.:BCC1647
CAS No.:914471-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 258282-23-4 | SDF | Download SDF |
| PubChem ID | 44561101 | Appearance | Powder |
| Formula | C17H30Br2N2 | M.Wt | 422.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 50 mM in DMSO | ||
| Chemical Name | N'-(1-phenylcyclohexyl)pentane-1,5-diamine;dihydrobromide | ||
| SMILES | C1CCC(CC1)(C2=CC=CC=C2)NCCCCCN.Br.Br | ||
| Standard InChIKey | ZCYUSVRXEKAQSL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H28N2.2BrH/c18-14-8-3-9-15-19-17(12-6-2-7-13-17)16-10-4-1-5-11-16;;/h1,4-5,10-11,19H,2-3,6-9,12-15,18H2;2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Voltage- and use-dependent open-channel antagonist of AMPA receptors. Selective between subtypes; blocks GluR2 subunit-lacking receptors more potently than GluR2-containing receptors (KD for GluR2-containing AMPAR is 210 times higher at -80 mV). More potent than IEM 1460 and IEM 1754 due to a slower unblocking rate. Alleviates inflammatory pain in a rat model of peripheral inflammation. |

IEM 1925 dihydrobromide Dilution Calculator

IEM 1925 dihydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3683 mL | 11.8416 mL | 23.6832 mL | 47.3664 mL | 59.208 mL |
| 5 mM | 0.4737 mL | 2.3683 mL | 4.7366 mL | 9.4733 mL | 11.8416 mL |
| 10 mM | 0.2368 mL | 1.1842 mL | 2.3683 mL | 4.7366 mL | 5.9208 mL |
| 50 mM | 0.0474 mL | 0.2368 mL | 0.4737 mL | 0.9473 mL | 1.1842 mL |
| 100 mM | 0.0237 mL | 0.1184 mL | 0.2368 mL | 0.4737 mL | 0.5921 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ghrelin (human)
Catalog No.:BCC7076
CAS No.:258279-04-8
- LEP (116-130) (mouse)
Catalog No.:BCC1016
CAS No.:258276-95-8
- Gemfibrozil
Catalog No.:BCC4783
CAS No.:25812-30-0
- Pelitinib (EKB-569)
Catalog No.:BCC1118
CAS No.:257933-82-7
- H-D-Glu(OBzl)-OH
Catalog No.:BCC2939
CAS No.:2578-33-8
- CP 154526
Catalog No.:BCC7481
CAS No.:257639-98-8
- J 104129 fumarate
Catalog No.:BCC7389
CAS No.:257603-40-0
- Velutin
Catalog No.:BCN5130
CAS No.:25739-41-7
- KF 38789
Catalog No.:BCC5938
CAS No.:257292-29-8
- Homoarbutin
Catalog No.:BCN2680
CAS No.:25712-94-1
- Tutin
Catalog No.:BCN5129
CAS No.:2571-22-4
- Lonicerin
Catalog No.:BCN8266
CAS No.:25694-72-8
- Ghrelin (rat)
Catalog No.:BCC5767
CAS No.:258338-12-4
- H-D-Cys(Trt)-OH
Catalog No.:BCC2914
CAS No.:25840-82-8
- Liensinine
Catalog No.:BCN6337
CAS No.:2586-96-1
- Hoechst 33258 analog
Catalog No.:BCC1624
CAS No.:258843-62-8
- Yunaconitoline
Catalog No.:BCN6703
CAS No.:259099-25-7
- D-Luciferin
Catalog No.:BCC6535
CAS No.:2591-17-5
- 3'-Hydroxydehydroaglaiastatin
Catalog No.:BCN7725
CAS No.:259143-58-3
- Boc-Thr-OH
Catalog No.:BCC3449
CAS No.:2592-18-9
- Boc-Lys(Boc)-ONp
Catalog No.:BCC3414
CAS No.:2592-19-0
- HOBt (anhydrous)
Catalog No.:BCC2816
CAS No.:2592-95-2
- EDC.HCl
Catalog No.:BCC2812
CAS No.:25952-53-8
- Allura Red AC
Catalog No.:BCN2220
CAS No.:25956-17-6
Inhibition of Spinal Ca(2+)-Permeable AMPA Receptors with Dicationic Compounds Alleviates Persistent Inflammatory Pain without Adverse Effects.[Pubmed:26973464]
Front Cell Neurosci. 2016 Feb 29;10:50.
Upregulation of Ca(2+)-permeable AMPA receptors (CP-AMPARs) in the dorsal horn (DH) neurons of the spinal cord has been causally linked to the maintenance of persistent inflammatory pain. Therefore, inhibition of CP-AMPARs could potentially alleviate an, otherwise, poorly treatable chronic pain. However, a loss of CP-AMPARs could produce considerable side effects because of the crucial role of CP-AMPARs in synaptic plasticity. Here we have tested whether the inhibition of spinal CP-AMPARs with dicationic compounds, the open-channel antagonists acting in an activity-dependent manner, can relieve inflammatory pain without adverse effects being developed. Dicationic compounds, N1-(1-phenylcyclohexyl)pentane-1,5-diaminium bromide (IEM-1925) and 1-trimethylammonio-5-1-adamantane-methyl-ammoniopentane dibromide (IEM-1460) were applied intrathecally (i.t.) as a post-treatment for inflammatory pain in the model of complete Freund's adjuvant (CFA)-induced long-lasting peripheral inflammation. The capability of dicationic compounds to ameliorate inflammatory pain was tested in rats in vivo using the Hargreaves, the von Frey and the open-field tests. Treatment with IEM-1460 or IEM-1925 resulted in profound alleviation of inflammatory pain. The pain relief appeared shortly after compound administration. The effects were concentration-dependent, displaying a high potency of dicationic compounds for alleviation of inflammatory hyperalgesia in the micromolar range, for both acute and long-lasting responses. The period of pain maintenance was shortened following treatment. Treatment with IEM-1460 or IEM-1925 changed neither thermal and mechanical basal sensitivities nor animal locomotion, suggesting that inhibition of CP-AMPARs with dicationic compounds does not give rise to detectable side effects. Thus, the ability of dicationic compounds to alleviate persistent inflammatory pain may provide new routes in the treatment of chronic pain.
Voltage-dependent block of native AMPA receptor channels by dicationic compounds.[Pubmed:10694232]
Br J Pharmacol. 2000 Jan;129(2):265-74.
1. The kinetics of open channel block of GluR2-containing and GluR2-lacking AMPA receptors (AMPAR) by dicationic compounds (IEM-1460, IEM-1754, and IEM-1925) have been studied in rat hippocampal neurones using whole-cell patch clamp recording and concentration-jump techniques. Neurones were isolated from hippocampal slices by vibrodissociation. 2. The dicationic compounds were approximately 100 - 200 times more potent as blockers of GluR2-lacking AMPAR than as blockers of GluR2-containing AMPAR. The subunit specificity of channel block is determined by the blocking rate constant of a dicationic compound, whereas differences in unblocking rate constants account for differences in potency. 3. Hyperpolarization may decrease the block produced by IEM-1460 and IEM-1754 block due to the voltage-dependence of the unblocking rate constants for these compounds. This suggests that dicationic compounds permeate the AMPAR channel at negative membrane potentials. The effect was particularly apparent for GluR2-lacking AMPAR. These findings indicate that the presence of GluR2-subunit(s) in AMPAR hinders the binding of the cationic compounds and their permeation through the channel. 4. The most potent compound tested was IEM-1925. The presence of a phenylcyclohexyl moiety instead of an adamantane moiety, as in IEM-1460 and IEM1754, is probably responsible for the higher potency of IEM-1925. Dicationic compounds are important not only as pharmacological tools, but also as templates for the synthesis of new selective AMPAR blockers which may be potential therapeutic agents.


