LEP (116-130) (mouse)An antiobesity hormone CAS# 258276-95-8 |

- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- Necrostatin 2 racemate
Catalog No.:BCC2077
CAS No.:852391-15-2
- Necrostatin 2
Catalog No.:BCC1793
CAS No.:852391-19-6
- Necrostatin 2 S enantiomer
Catalog No.:BCC2078
CAS No.:852391-20-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 258276-95-8 | SDF | Download SDF |
| PubChem ID | 90471193 | Appearance | Powder |
| Formula | C64H109N19O24S | M.Wt | 1560.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 50 mg/mL (32.04 mM) *"≥" means soluble, but saturation unknown. | ||
| Sequence | SCSLPQTSGLQKPES (Modifications: Ser-15 = C-terminal amide) | ||
| Chemical Name | (4S)-4-[[(2S)-1-[(2S)-6-amino-2-[[(2S)-5-amino-2-[[(2S)-2-[[2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-5-amino-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2S)-2-amino-3-hydroxypropanoyl]amino]-3-sulfanylpropanoyl]amino]-3-hydroxypropanoyl]amino]-4-methylpentanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-3-hydroxybutanoyl]amino]-3-hydroxypropanoyl]amino]acetyl]amino]-4-methylpentanoyl]amino]-5-oxopentanoyl]amino]hexanoyl]pyrrolidine-2-carbonyl]amino]-5-[[(2S)-1-amino-3-hydroxy-1-oxopropan-2-yl]amino]-5-oxopentanoic acid | ||
| SMILES | CC(C)CC(C(=O)NC(CCC(=O)N)C(=O)NC(CCCCN)C(=O)N1CCCC1C(=O)NC(CCC(=O)O)C(=O)NC(CO)C(=O)N)NC(=O)CNC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C(CCC(=O)N)NC(=O)C2CCCN2C(=O)C(CC(C)C)NC(=O)C(CO)NC(=O)C(CS)NC(=O)C(CO)N | ||
| Standard InChIKey | YEHDWBRMEXDYLT-RDEOYNLOSA-N | ||
| Standard InChI | InChI=1S/C64H109N19O24S/c1-30(2)22-38(57(100)72-34(13-16-46(67)89)54(97)75-37(10-6-7-19-65)63(106)82-20-8-11-44(82)60(103)74-36(15-18-49(92)93)55(98)77-40(26-85)51(69)94)71-48(91)24-70-53(96)41(27-86)79-62(105)50(32(5)88)81-56(99)35(14-17-47(68)90)73-61(104)45-12-9-21-83(45)64(107)39(23-31(3)4)76-58(101)42(28-87)78-59(102)43(29-108)80-52(95)33(66)25-84/h30-45,50,84-88,108H,6-29,65-66H2,1-5H3,(H2,67,89)(H2,68,90)(H2,69,94)(H,70,96)(H,71,91)(H,72,100)(H,73,104)(H,74,103)(H,75,97)(H,76,101)(H,77,98)(H,78,102)(H,79,105)(H,80,95)(H,81,99)(H,92,93)/t32-,33+,34+,35+,36+,37+,38+,39+,40+,41+,42+,43+,44+,45+,50+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Synthetic leptin peptide fragment. Restricts weight gain and reduces food intake in female mice lacking endogenously circulating active leptin. |

LEP (116-130) (mouse) Dilution Calculator

LEP (116-130) (mouse) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Leptin (116-130), amide, mouse(C64H109N19O24S),a peptide with the sequence Ser-Cys-Ser-Leu-Pro-Gln-Thr-Ser-Gly-Leu-Gln-Lys-Pro-Glu-Ser-NH2. Leptin is an adipocyte-derived hormone that acts as a major regulator for food intake and energy homeostasis. Leptin deficiency or resistance can result in profound obesity, diabetes, and infertility in humans. Since the discovery of leptin, the breadth of biological actions has dramatically expanded and served to broaden the initial perspective, where this protein was viewed solely as an antiobesity hormone. Important biological activities have been discovered in peripheral tissues that demonstrate the pleiotropic effects of this molecule in such areas as hematopoiesis, angiogenesis, blood pressure, bone mass, lymphoid organ homeostasis, and T lymphocyte function. Recent data indicate that leptin(116-130), an active fragment of the native molecule, exerts effects similar to those of the native peptide on body weight and food intake.
Figure1 Formula of Leptin (116-130)
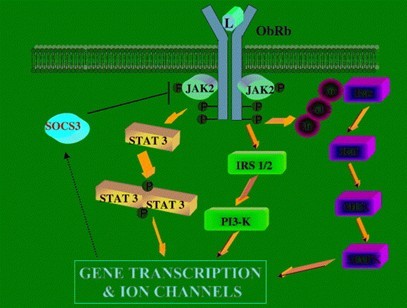
Figure2 Signal transduction pathways of leptin
Ref:
1. Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, Schoner BE, Smith DP, Zhang XY, Wery JP, Schevitz RW (May 1997). "Crystalstructure of the obese protein leptin-E100". Nature 387 (6629): 206–9.
2. Brennan AM, Mantzoros CS (June 2006). "Drug Insight: the role of leptin in human physiology and pathophysiology--emerging clinical applications". Nat Clin Pract Endocrinol Metab 2 (6): 318–327.
3. GreGreen ED, Maffei M, Braden VV, Proenca R, DeSilva U, Zhang Y, Chua SC Jr, Leibel RL, Weissenbach J, Friedman JM (August 1995). "The human obese (OB) gene: RNA expression pattern and mapping on the physical, cytogenetic, and genetic maps of chromosome 7". Genome Res. 5 (1): 5–12.
4. Margetic S, Gazzola C, Pegg GG, Hill RA (2002). "Leptin: a review of its peripheral actions and interactions". Int. J. Obes. Relat. Metab. Disord. 26 (11): 1407–1433.
- Gemfibrozil
Catalog No.:BCC4783
CAS No.:25812-30-0
- Pelitinib (EKB-569)
Catalog No.:BCC1118
CAS No.:257933-82-7
- H-D-Glu(OBzl)-OH
Catalog No.:BCC2939
CAS No.:2578-33-8
- CP 154526
Catalog No.:BCC7481
CAS No.:257639-98-8
- J 104129 fumarate
Catalog No.:BCC7389
CAS No.:257603-40-0
- Velutin
Catalog No.:BCN5130
CAS No.:25739-41-7
- KF 38789
Catalog No.:BCC5938
CAS No.:257292-29-8
- Homoarbutin
Catalog No.:BCN2680
CAS No.:25712-94-1
- Tutin
Catalog No.:BCN5129
CAS No.:2571-22-4
- Lonicerin
Catalog No.:BCN8266
CAS No.:25694-72-8
- HU 308
Catalog No.:BCC5971
CAS No.:256934-39-1
- Cimiracemoside C
Catalog No.:BCN5128
CAS No.:256925-92-5
- Ghrelin (human)
Catalog No.:BCC7076
CAS No.:258279-04-8
- IEM 1925 dihydrobromide
Catalog No.:BCC7885
CAS No.:258282-23-4
- Ghrelin (rat)
Catalog No.:BCC5767
CAS No.:258338-12-4
- H-D-Cys(Trt)-OH
Catalog No.:BCC2914
CAS No.:25840-82-8
- Liensinine
Catalog No.:BCN6337
CAS No.:2586-96-1
- Hoechst 33258 analog
Catalog No.:BCC1624
CAS No.:258843-62-8
- Yunaconitoline
Catalog No.:BCN6703
CAS No.:259099-25-7
- D-Luciferin
Catalog No.:BCC6535
CAS No.:2591-17-5
- 3'-Hydroxydehydroaglaiastatin
Catalog No.:BCN7725
CAS No.:259143-58-3
- Boc-Thr-OH
Catalog No.:BCC3449
CAS No.:2592-18-9
- Boc-Lys(Boc)-ONp
Catalog No.:BCC3414
CAS No.:2592-19-0
- HOBt (anhydrous)
Catalog No.:BCC2816
CAS No.:2592-95-2
Inhibitory effects of leptin-related synthetic peptide 116-130 on food intake and body weight gain in female C57BL/6J ob/ob mice may not be mediated by peptide activation of the long isoform of the leptin receptor.[Pubmed:10535455]
Diabetes. 1999 Nov;48(11):2204-9.
We recently reported that intraperitoneal administration of leptin-related synthetic peptide 116-130 [LEP-(116-130)] resulted in reduced food intake and significant weight loss in homozygous female C57BL/6J ob/ob mice. In this study, we used two in vitro bioassays to show that the interaction of LEP-(116-130) with the long form of the leptin receptor (OB-Rb), the receptor isoform that is predominantly expressed in the hypothalamus, is not required for the observed in vivo effects of the peptide on energy balance. LEP-(116-130) was unable to compete the binding of alkaline phosphatase-leptin fusion protein to OB-R. Moreover, LEP-(116-130) was unable to activate signal transduction by OB-Rb in vitro. In homozygous female C57BLKS/J-m db/db mice that do not express OB-Rb, intraperitoneal administration of LEP-(116-130) reduced body weight gain and blood glucose levels but not food intake, which further supports a mechanism of action that does not require peptide interaction with OB-Rb.
Design of a synthetic leptin agonist: effects on energy balance, glucose homeostasis, and thermoregulation.[Pubmed:10875251]
Endocrinology. 2000 Jul;141(7):2501-7.
We have previously reported that a synthetic peptide amide corresponding to amino acid residues 116-130 of mouse leptin, LEP-(116-130), reduces body weight gain, food intake, and blood glucose levels in ob/ob and db/db mice. In the present study we show that the activity of LEP-(116-130) resides in a restricted sequence between amino acid residues 116-122. A synthetic peptide corresponding to this sequence (Ser-Cys-Ser-Leu-Pro-Gln-Thr) has been named OB3. Single point D-amino acid substitution was used to study the structure-function relationship of each residue in OB3. D-Amino acid analogs of OB3 were synthesized by the solid phase method, purified to 98+%, and administered (1 mg/day, ip) for 7 days to female C57BL/6J ob/ob mice. The effects of the peptides on body weight gain, food and water intake, glucose homeostasis, and thermoregulation were assessed. In most cases, the efficacy of OB3 on all parameters tested was reduced by substitution of an L-amino acid with its corresponding D-isoform. A statistically significant increase (2.6-fold) in the weight-reducing effect of OB3, however, was observed by inversion of the configuration of the leucine residue at position 4 (Leu-4) of OB3 by substitution with its D-amino acid isoform [D-Leu-4]. Compared with OB3, mice treated with [D-Leu-4]-OB3 consumed 7.9% less food and 16.5% less water. Blood glucose was normalized to levels comparable to those in wild-type control mice within 2 days after initiation of [D-Leu-4]-OB3 treatment. Unlike native leptin, however, neither OB3 nor any of its D-amino acid-substituted analogs had any apparent effect on thermogenesis. Our results indicate that synthetic peptide strategies may be useful in the development of potent and stabile pharmacophores with potential therapeutic significance in the treatment of human obesity and its related metabolic dysfunctions.
In vivo effects of leptin-related synthetic peptides on body weight and food intake in female ob/ob mice: localization of leptin activity to domains between amino acid residues 106-140.[Pubmed:9075696]
Endocrinology. 1997 Apr;138(4):1413-8.
In C57BL/6J ob/ob mice, a single base mutation of the ob gene in codon 105 results in the replacement of arginine by a premature stop codon and production of a truncated inactive form of leptin. These observations suggest that leptin activity may be localized, at least in part, to domains distal to amino acid residue 104. To investigate this possibility, we synthesized six overlapping peptide amides corresponding to residues 106-167 of leptin, and examined their effects on body weight and food intake in female C57BL/6J ob/ob mice. When compared with vehicle-injected control mice, weight gain by mice receiving 28 daily 1-mg i.p. injections of LEP-(106-120), LEP-(116-130), or LEP-(126-140) was significantly (P < 0.01) reduced with no apparent toxicity. Weight gain by mice receiving LEP-(136-150), LEP-(146-160), or LEP-(156-167) was not significantly different from that of vehicle-injected control mice. The effects of LEP-(106-120), LEP-(116-130), or LEP-(126-140) were most pronounced during the first week of peptide treatment. Within 7 days, mice receiving these peptides lost 12.3%, 13.8%, and 9.8%, respectively, of their initial body weights. After 28 days, mice given vehicle alone, LEP-(136-150), LEP-(146-160), or LEP-(156-167) were 14.7%, 20.3%, 25.0%, and 24.8% heavier, respectively, than they were at the beginning of the study. Mice given LEP-(106-120) or LEP-(126-140) were only 1.8% and 4.2% heavier, respectively, whereas mice given LEP-(116-130) were 3.4% lighter. Food intake by mice receiving LEP-(106-120), LEP-(116-130), or LEP-(126-140), but not by mice receiving LEP-(136-150), LEP-(146-160), or LEP-(156-167), was reduced by 15%. The results of this study indicate 1) that leptin activity is localized, at least in part, in domains between residues 106-140; 2) that leptin-related peptides have in vivo effects similar to those of native leptin; and 3) offer hope for development of peptide analogs of leptin having potential application in human or veterinary medicine.


