Ghrelin (human)Endogenous ligand for the GHS receptor CAS# 258279-04-8 |
- Pepstatin A
Catalog No.:BCC1218
CAS No.:26305-03-3
- Vicriviroc Malate
Catalog No.:BCC1230
CAS No.:541503-81-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 258279-04-8 | SDF | Download SDF |
| PubChem ID | 44134733 | Appearance | Powder |
| Formula | C149H249N47O42 | M.Wt | 3370.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 2 mg/ml in water | ||
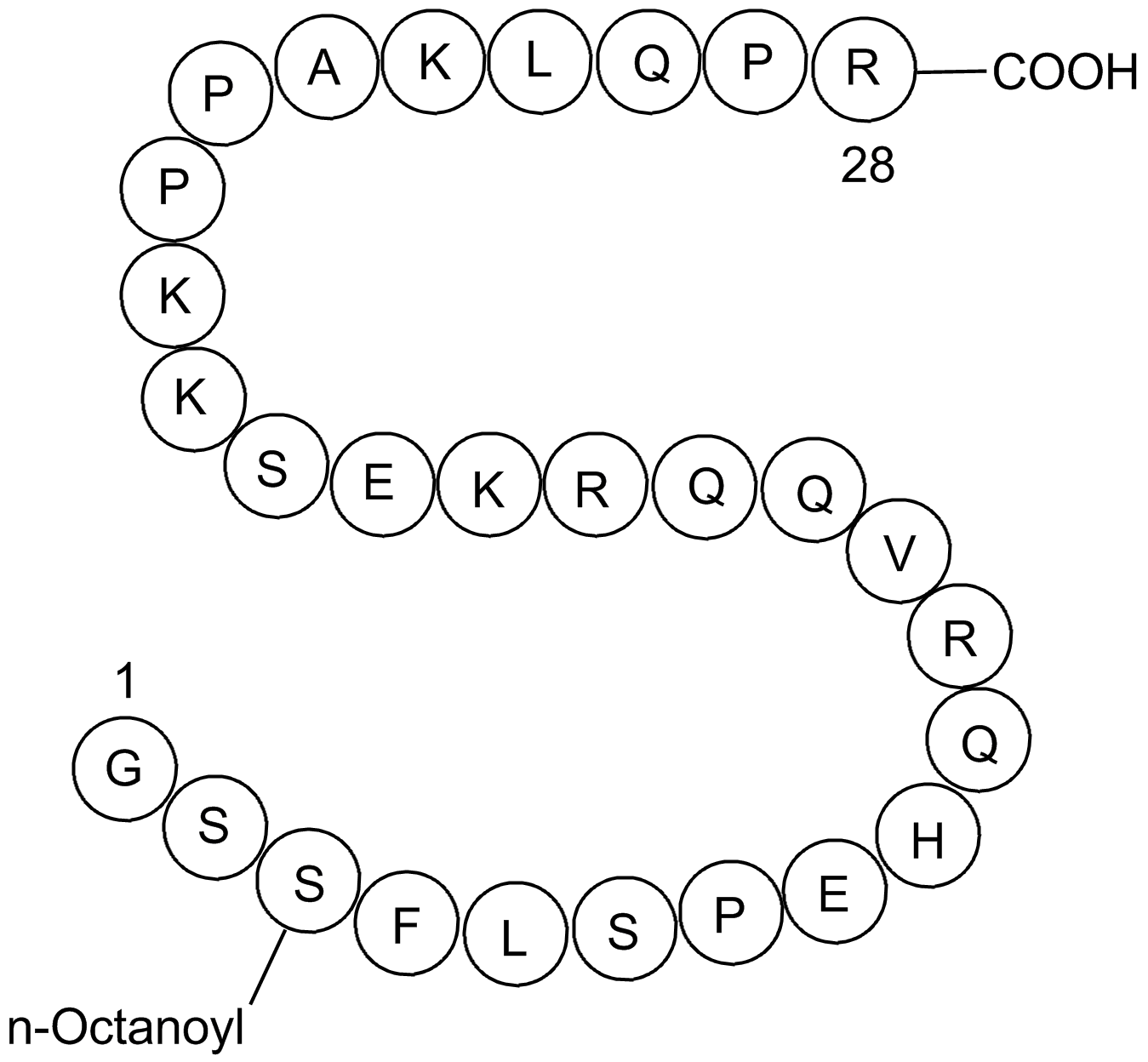
| Sequence | GSSFLSPEHQRVQQRKESKKPPAKLQPR (Modifications: Ser-3 = Ser(n-octanoyl)) | ||
| Chemical Name | 4-[[1-[2-[[2-[[2-[[2-[[2-[(2-aminoacetyl)amino]-3-hydroxypropanoyl]-octanoylamino]-3-hydroxypropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]-3-hydroxypropanoyl]pyrrolidine-2-carbonyl]amino]-5-[[1-[[5-amino-1-[[1-[[1-[[5-amino-1-[[5-amino-1-[[1-[[6-amino-1-[[1-[[1-[[6-amino-1-[[6-amino-1-[2-[2-[[1-[[6-amino-1-[[1-[[5-amino-1-[2-[(4-carbamimidamido-1-carboxybutyl)carbamoyl]pyrrolidin-1-yl]-1,5-dioxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxopropan-2-yl]carbamoyl]pyrrolidine-1-carbonyl]pyrrolidin-1-yl]-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-1-oxohexan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl]amino]-5-oxopentanoic acid | ||
| SMILES | CCCCCCCC(=O)N(C(CO)C(=O)NC(CC1=CC=CC=C1)C(=O)NC(CC(C)C)C(=O)NC(CO)C(=O)N2CCCC2C(=O)NC(CCC(=O)O)C(=O)NC(CC3=CNC=N3)C(=O)NC(CCC(=O)N)C(=O)NC(CCCNC(=N)N)C(=O)NC(C(C)C)C(=O)NC(CCC(=O)N)C(=O)NC(CCC(=O)N)C(=O)NC(CCCNC(=N)N)C(=O)NC(CCCCN)C(=O)NC(CCC(=O)O)C(=O)NC(CO)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(=O)N4CCCC4C(=O)N5CCCC5C(=O)NC(C)C(=O)NC(CCCCN)C(=O)NC(CC(C)C)C(=O)NC(CCC(=O)N)C(=O)N6CCCC6C(=O)NC(CCCNC(=N)N)C(=O)O)C(=O)C(CO)NC(=O)CN | ||
| Standard InChIKey | QPONSPOGTFKFDV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C149H249N47O42/c1-9-10-11-12-16-45-116(206)196(144(235)105(77-199)171-115(205)73-154)110(78-200)139(230)188-101(71-84-32-14-13-15-33-84)133(224)186-100(70-81(4)5)132(223)190-104(76-198)143(234)193-66-29-42-107(193)137(228)180-95(51-57-118(209)210)128(219)187-102(72-85-74-165-79-169-85)134(225)179-92(47-53-112(156)202)126(217)175-90(39-26-63-167-148(161)162)130(221)191-119(82(6)7)140(231)181-93(48-54-113(157)203)127(218)177-91(46-52-111(155)201)125(216)174-89(38-25-62-166-147(159)160)122(213)173-87(35-18-22-59-151)121(212)178-94(50-56-117(207)208)129(220)189-103(75-197)135(226)176-88(36-19-23-60-152)123(214)182-96(37-20-24-61-153)141(232)195-68-31-44-109(195)145(236)194-67-30-41-106(194)136(227)170-83(8)120(211)172-86(34-17-21-58-150)124(215)185-99(69-80(2)3)131(222)183-97(49-55-114(158)204)142(233)192-65-28-43-108(192)138(229)184-98(146(237)238)40-27-64-168-149(163)164/h13-15,32-33,74,79-83,86-110,119,197-200H,9-12,16-31,34-73,75-78,150-154H2,1-8H3,(H2,155,201)(H2,156,202)(H2,157,203)(H2,158,204)(H,165,169)(H,170,227)(H,171,205)(H,172,211)(H,173,213)(H,174,216)(H,175,217)(H,176,226)(H,177,218)(H,178,212)(H,179,225)(H,180,228)(H,181,231)(H,182,214)(H,183,222)(H,184,229)(H,185,215)(H,186,224)(H,187,219)(H,188,230)(H,189,220)(H,190,223)(H,191,221)(H,207,208)(H,209,210)(H,237,238)(H4,159,160,166)(H4,161,162,167)(H4,163,164,168) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous agonist peptide for the ghrelin receptor (GHS-R1a). Produced mainly by the stomach, it stimulates release of growth hormone from the pituitary gland in vitro and in vivo, and regulates feeding, growth and energy production. |

Ghrelin (human) Dilution Calculator

Ghrelin (human) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ghrelin is a specific endogenous ligand for the GHS receptor which can stimulate strong increase in circulating GH levels both in vitro and in vivo in a dose-dependent manner, Human acylated ghrelin is the major active form that can cross the blood-brain barrier, and has an effection on the activity of arcute neurones with a much stronger affinity (IC50, 0.3×10-9M) than GHSR antagonist (D-Lys3)-GHRP-6 (IC50, 0.9×10-6M)[1].
Ghrelin has effects in stimulating appetite, increasing secretion of GH and hypo-catabolism, improvement of gastrointestinal motility, increasing cardiac output, improvement of pulmonary function and anti-inflammatory effect.
Clinical applications of ghrelin in total gastrectomy, esophagectomy and neoadjuvant chemotherapy can stimulate food intake, improve guality of life scores and minimize adverse events. Recently, ghrelin as a effective approach in the management of anorexia nervosa[2],cachexia[3], BW[4] and appetite loss[5], systemic inflammatory response[6]and cancer[7]. In conclusion, ghrelin is a promising candidate for treating catabolic states and enhancing immune function in cachexia or acquired immunodeficiency syndrome, as well as for treating eating disorders such as obesity and anorexia nervosa.
References:
1. M. Traebert,* T. Riediger,† S. Whitebread,* E. Scharrer† and H. A. Schmid*. Ghrelin Acts on Leptin-Responsive Neurones in the Rat Arcuate Nucleus. Journal of Neuroendocrinology, 2002, Vol. 14, 580–586
2. Hotta M, Ohwada R, Akamizu T, Shibasaki T, Takano K, Kangawa K. Ghrelin increases hunger and food intake in patients with restricting-type anorexia nervosa: a pilot study. Endocr J. 2009;56:1119–28.
3. Nagaya N, Itoh T, Murakami S, Oya H, Uematsu M, Miyatake K, et al. Treatment of cachexia with ghrelin in patients with COPD. Chest. 2005;128:1187–93.
4. Adachi S, Takiguchi S, Okada K, Yamamoto K, Yamasaki M, Miyata H, et al. Effects of ghrelin administration after total gastrectomy: a prospective, randomized, placebo-controlled phase II study. Gastroenterology. 2010;138:1312–20.
5. Hiura Y, Takiguchi S, Yamamoto K, Kurokawa Y, Yamasaki M, Nakajima K, et al. Fall in plasma ghrelin concentrations after cisplatin-based chemotherapy in esophageal cancer patients. Int J Clin Oncol. 2012;17:316–23.
6. Cheyuo C, Jacob A, Wang P. Ghrelin-mediated sympathoinhibition and suppression of inflammation in sepsis. Am J Physiol Endocrinol Metab. 2012;302:E265–72.
7. Yoshida N, Watanabe M, Baba Y, Iwagami S, Ishimoto T, Iwatsuki M, et al. Risk factors for pulmonary complications after esophagectomy for esophageal cancer. Surg Today. 2014;44:526–32.
- LEP (116-130) (mouse)
Catalog No.:BCC1016
CAS No.:258276-95-8
- Gemfibrozil
Catalog No.:BCC4783
CAS No.:25812-30-0
- Pelitinib (EKB-569)
Catalog No.:BCC1118
CAS No.:257933-82-7
- H-D-Glu(OBzl)-OH
Catalog No.:BCC2939
CAS No.:2578-33-8
- CP 154526
Catalog No.:BCC7481
CAS No.:257639-98-8
- J 104129 fumarate
Catalog No.:BCC7389
CAS No.:257603-40-0
- Velutin
Catalog No.:BCN5130
CAS No.:25739-41-7
- KF 38789
Catalog No.:BCC5938
CAS No.:257292-29-8
- Homoarbutin
Catalog No.:BCN2680
CAS No.:25712-94-1
- Tutin
Catalog No.:BCN5129
CAS No.:2571-22-4
- Lonicerin
Catalog No.:BCN8266
CAS No.:25694-72-8
- HU 308
Catalog No.:BCC5971
CAS No.:256934-39-1
- IEM 1925 dihydrobromide
Catalog No.:BCC7885
CAS No.:258282-23-4
- Ghrelin (rat)
Catalog No.:BCC5767
CAS No.:258338-12-4
- H-D-Cys(Trt)-OH
Catalog No.:BCC2914
CAS No.:25840-82-8
- Liensinine
Catalog No.:BCN6337
CAS No.:2586-96-1
- Hoechst 33258 analog
Catalog No.:BCC1624
CAS No.:258843-62-8
- Yunaconitoline
Catalog No.:BCN6703
CAS No.:259099-25-7
- D-Luciferin
Catalog No.:BCC6535
CAS No.:2591-17-5
- 3'-Hydroxydehydroaglaiastatin
Catalog No.:BCN7725
CAS No.:259143-58-3
- Boc-Thr-OH
Catalog No.:BCC3449
CAS No.:2592-18-9
- Boc-Lys(Boc)-ONp
Catalog No.:BCC3414
CAS No.:2592-19-0
- HOBt (anhydrous)
Catalog No.:BCC2816
CAS No.:2592-95-2
- EDC.HCl
Catalog No.:BCC2812
CAS No.:25952-53-8
Development of ghrelin resistance in a cancer cachexia rat model using human gastric cancer-derived 85As2 cells and the palliative effects of the Kampo medicine rikkunshito on the model.[Pubmed:28249026]
PLoS One. 2017 Mar 1;12(3):e0173113.
Cancer cachexia (CC) is a multifactorial disease characterized by decreased food intake and loss of body weight due to reduced musculature with or without loss of fat mass. Patients with gastric cancer have a high incidence of cachexia. We previously established a novel CC rat model induced by human gastric cancer-derived 85As2 cells in order to examine the pathophysiology of CC and identify potential therapeutics. In patients with CC, anorexia is often observed, despite elevation of ghrelin, suggesting that ghrelin resistance may develop in these patients. In this study, we aimed to clarify the occurrence of ghrelin resistance in CC rats accompanied by anorexia and we investigated whether rikkunshito (RKT), a traditional Japanese Kampo medicine that potentiates ghrelin signaling, ameliorated CC-related anorexia through alleviation of ghrelin resistance. 85As2-tumor-bearing rats developed severe CC symptoms, including anorexia and loss of body weight/musculature, with the latter symptoms being greater in cachectic rats than in non-tumor-bearing or pair-fed rats. CC rats showed poor responses to intraperitoneal injection of ghrelin. In CC rats, plasma ghrelin levels were elevated and hypothalamic anorexigenic peptide mRNA levels were decreased, whereas hypothalamic growth hormone secretagogue receptor (GHS-R) mRNA was not affected. In vitro, RKT directly enhanced ghrelin-induced GHS-R activation. RKT administrated orally for 7 days partly alleviated the poor response to ghrelin and ameliorated anorexia without affecting the elevation of plasma ghrelin levels in CC rats. The expression of hypothalamic orexigenic neuropeptide Y mRNA but not hypothalamic GHS-R mRNA was increased by RKT. Thus, the 85As2 cell-induced CC rat model developed ghrelin resistance, possibly contributing to anorexia and body weight loss. The mechanism through which RKT ameliorated anorexia in the CC rat model may involve alleviation of ghrelin resistance by enhancement of ghrelin signaling. These findings suggest that RKT may be a promising agent for the treatment of CC.
Therapeutic effect of human ghrelin and growth hormone: Attenuation of immunosuppression in septic aged rats.[Pubmed:28115288]
Biochim Biophys Acta Mol Basis Dis. 2017 Oct;1863(10 Pt B):2584-2593.
Sepsis is a leading cause of mortality in intensive care units, and is more common in the geriatric population. The control of hyperinflammation has been suggested as a therapeutic approach in sepsis, but to date clinical trials utilizing this strategy have not lead to an effective treatment. In addition to hyperinflammation, patients with sepsis often experience a state of immunosuppression, which serves as an important determinant for increased morbidity and mortality. We previously used aged animals to demonstrate the effectiveness of combined treatment with human ghrelin (Ghr) and human growth hormone (GH) in improving organ injury and survival in septic animals. Here, we hypothesized that combined treatment with Ghr and GH could improve immune function in septic aged animals. Male 24-month-old rats were subjected to cecal ligation and puncture (CLP) for sepsis induction. Human Ghr (80nmol/kg BW) plus GH (50mug/kg BW) or vehicle (normal saline) was administrated subcutaneously at 5h after CLP. The ex vivo production of TNF-alpha, IL-6 and IL-10 to LPS-stimulation, as well as TNF-alpha, IL-6, IL-10 and IFN-gamma production to anti-CD3/anti-CD28 antibody-stimulation, in splenocytes isolated 20h after CLP, was significantly decreased compared to production of these cytokines in splenocytes from sham animals. The production of cytokines from splenocytes isolated from septic animals that received the combined treatment, however, was significantly higher than from those isolated from vehicle-treated septic animals. Combined treatment prevented the loss of splenic CD4(+) and CD8(+) T cells in septic aged rats, and reduced lymphocyte apoptosis. Combined treatment also inhibited an increase in the regulatory T cell (Treg) population and expression of the immune co-inhibitory molecule PD-1 in the spleens of septic aged rats. In contrast, expression of HLA-DR was increased after combined treatment with Ghr and GH. Based on these findings, we conclude that co-administration of Ghr and GH is a promising therapeutic tool for reversing immunosuppression caused by sepsis in the geriatric population. This article is part of a Special Issue entitled: Immune and Metabolic Alterations in Trauma and Sepsis edited by Dr. Raghavan Raju.
Expression of ghrelin and ghrelin functional receptor GHSR1a in human pituitary adenomas.[Pubmed:28377557]
Pol Arch Intern Med. 2017 Mar 31;127(3):163-169.
INTRODUCTION Pituitary adenomas are heterogenous lesions commonly observed in the central nervous system. Signal transduction of ghrelin, an endogenous ligand specific for growth hormone secretagogue receptor (GHSR), has been reported to be involved in the development of endocrine tumors. However, there are limited data concerning the role of ghrelin and its functional receptor in pituitary adenomas. OBJECTIVES The aim of the study was to establish the expression pattern of GHRL and its functional receptor GHSR1a in human pituitary adenomas. PATIENTS AND METHODS Tissue specimens, including somatotropinomas (n = 20), prolactinomas (n = 5), and nonfunctioning adenomas (n = 52) were obtained from 77 patients. Thirteen normal pituitaries served as controls. The expression pattern of GHRL and GHSR1a mRNAs was established using reverse transcription followed by quantitative polymerase chain reaction. RESULTS Ghrelin mRNA was detected in 92.2% of the samples including controls, while GHSR1a transcripts were detected in 54.4% of the cases. Significant differences were found among subgroups in the GHSR1a expression (P <0.0001) but not in that of GHRL (P = 0.7). The relative GHSR1a expression level was significantly lower for nonfunctioning tumors than for the control group or somatotropinomas. Controls revealed a strong positive correlation between the expression of both genes (r = 0.8; P <0.0001), unlike adenomas, which showed a weak negative correlation (r = -0.3; P >0.05). The maximum tumor diameter for nonfunctioning adenomas was higher than that for somatotropinomas (mean [SD], 31.4 [76] mm vs 24.8 [10.9] mm; P = 0.01). Neither the GHRL nor GHSR1a expression showed a significant correlation with tumor size in the subgroups. CONCLUSIONS The presence of GHRL and GHSR1a in the neural system indicates their effect on pituitary function regulation and suggests their possible role in adenoma pathogenesis.
Synthetic Triterpenoid Inhibition of Human Ghrelin O-Acyltransferase: The Involvement of a Functionally Required Cysteine Provides Mechanistic Insight into Ghrelin Acylation.[Pubmed:28134508]
Biochemistry. 2017 Feb 21;56(7):919-931.
The peptide hormone ghrelin plays a key role in regulating hunger and energy balance within the body. Ghrelin signaling presents a promising and unexploited target for development of small molecule therapeutics for treatment of obesity, diabetes, and other health conditions. Inhibition of ghrelin O-acyltransferase (GOAT), which catalyzes an essential octanoylation step in ghrelin maturation, offers a potential avenue for controlling ghrelin signaling. Through screening a small molecule library, we have identified a class of synthetic triterpenoids that efficiently inhibit ghrelin acylation by the human isoform of GOAT (hGOAT). These compounds function as covalent reversible inhibitors of hGOAT, providing the first evidence of the involvement of a nucleophilic cysteine residue in substrate acylation by a MBOAT family acyltransferase. Surprisingly, the mouse form of GOAT does not exhibit susceptibility to cysteine-modifying electrophiles, revealing an important distinction in the activity and behavior between these closely related GOAT isoforms. This study establishes these compounds as potent small molecule inhibitors of ghrelin acylation and provides a foundation for the development of novel hGOAT inhibitors as therapeutics targeting diabetes and obesity.
In vivo and in vitro effects of ghrelin/motilin-related peptide on growth hormone secretion in the rat.[Pubmed:11174017]
Neuroendocrinology. 2001 Jan;73(1):54-61.
Ghrelin (Ghr), a 28 amino acid gastric peptide with an n-octanoylation on Ser 3, has recently been identified as an endogenous ligand of the growth hormone secretagogue (GHS) receptor. A cDNA was also isolated from a mouse stomach library encoding a protein named prepromotilin-related peptide (ppMTLRP) which shares sequence similarities with prepromotilin. Mouse and rat ppMTLRP sequences (rGhr) are identical and show 89% identity with human ghrelin (hGhr). By analogy with promotilin, cleavage of proMTLRP into an 18 amino acid endogenous processed peptide can be assumed on the basis of a conserved dibasic motif in position 9-10 of its sequence. In the present work, we compared the GH-releasing activity of rGhr28/MTLRP and of hGhr28/MTRLP with that of a shorter form of the peptide, hGhr18. A short peptide devoid of Ser-3 n-octanoylation hGhr18[-] was also tested. Addition of rGhr28, hGhr28 and hGhr18 stimulated GH release to the same extent from superfused pituitaries. The effect was dose dependent in a 10(-8) to 10(-6) M concentration range. In contrast, hGhr 18[-] was inactive. In freely moving animals, both rGhr28 and hGhr28 (10 microg, i.v.) stimulated GH release, whereas the same dose of hGhr18 or of hGhr18[-] was ineffective. After rGhr28, GH plasma levels increased as early as 5 min after injection and returned to basal values within 40-60 min. Expressed as percent stimulation, administration of rGhr28 was equally effective when injected during troughs or peaks of GH. Plasma concentrations of prolactin, adrenocorticotropin and leptin were not modified. Spontaneous GH secretory episodes were no longer observed within 3 h of rGhr28 treatment, but repeated administration of the secretagogue at 3- to 4-hour intervals resulted in a similar GH response. Activation of somatostatin (SRIH) release by ether stress did not blunt the GH response to rGhr28. This suggests that the secretagogue acts in part by inhibiting endogenous SRIH, as further substantiated by the ability of rGhr28 (10(-6) M), to decrease the amplitude of 25 mM K+-induced SRIH release from perifused hypothalami. In conclusion, (1) n-octanoylation of Ghrs and the shorter form hGhr18 is essential for the direct pituitary GH-releasing effect of this new family of endogenous GHSs; (2) only the longer forms are active in vivo and (3) inhibition of SRIH release appears involved in the mechanism of Ghr action.
Ghrelin is a growth-hormone-releasing acylated peptide from stomach.[Pubmed:10604470]
Nature. 1999 Dec 9;402(6762):656-60.
Small synthetic molecules called growth-hormone secretagogues (GHSs) stimulate the release of growth hormone (GH) from the pituitary. They act through GHS-R, a G-protein-coupled receptor for which the ligand is unknown. Recent cloning of GHS-R strongly suggests that an endogenous ligand for the receptor does exist and that there is a mechanism for regulating GH release that is distinct from its regulation by hypothalamic growth-hormone-releasing hormone (GHRH). We now report the purification and identification in rat stomach of an endogenous ligand specific for GHS-R. The purified ligand is a peptide of 28 amino acids, in which the serine 3 residue is n-octanoylated. The acylated peptide specifically releases GH both in vivo and in vitro, and O-n-octanoylation at serine 3 is essential for the activity. We designate the GH-releasing peptide 'ghrelin' (ghre is the Proto-Indo-European root of the word 'grow'). Human ghrelin is homologous to rat ghrelin apart from two amino acids. The occurrence of ghrelin in both rat and human indicates that GH release from the pituitary may be regulated not only by hypothalamic GHRH, but also by ghrelin.


