Pepstatin AAspartic proteinases inhibitor CAS# 26305-03-3 |
- VTP-27999 Hydrochloride
Catalog No.:BCC2050
CAS No.:1264191-73-2
- Aliskiren Hemifumarate
Catalog No.:BCC5018
CAS No.:173334-58-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 26305-03-3 | SDF | Download SDF |
| PubChem ID | 5462227 | Appearance | Powder |
| Formula | C34H63N5O9 | M.Wt | 685.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Pepstatin A | ||
| Solubility | DMSO : ≥ 25 mg/mL (36.45 mM) *"≥" means soluble, but saturation unknown. | ||
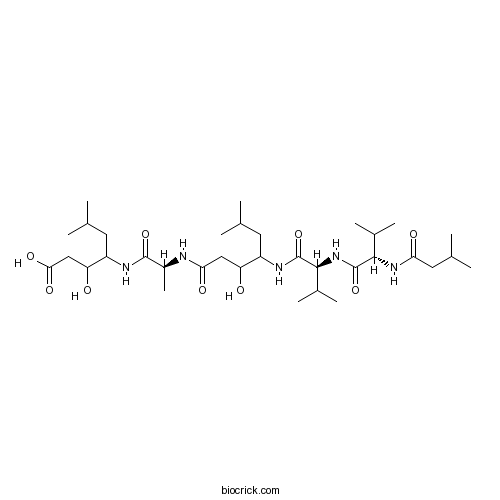
| Sequence | VVA (Modifications: Val-1 = N-terminal H-iso-Valeryl, Val-2 = Val-4-amino-3-hydroxy-6-methyl | ||
| Chemical Name | 3-hydroxy-4-[[(2S)-2-[[3-hydroxy-6-methyl-4-[[(2S)-3-methyl-2-[[(2S)-3-methyl-2-(3-methylbutanoylamino)butanoyl]amino]butanoyl]amino]heptanoyl]amino]propanoyl]amino]-6-methylheptanoic acid | ||
| SMILES | CC(C)CC(C(CC(=O)O)O)NC(=O)C(C)NC(=O)CC(C(CC(C)C)NC(=O)C(C(C)C)NC(=O)C(C(C)C)NC(=O)CC(C)C)O | ||
| Standard InChIKey | FAXGPCHRFPCXOO-JHTFMCFMSA-N | ||
| Standard InChI | InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23?,24?,25?,26?,30-,31-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Irreversible inhibitor of aspartic proteases. Inhibits lysosomal proteases and interferes with autolysosomal digestion when used in combination with E 64d. |

Pepstatin A Dilution Calculator

Pepstatin A Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pepstatin A is a well-known inhibitor of aspartic proteinases with IC50 values of 15 μM, 2 μM, < 5 nM and < 40 nM for human renin, HIV protease, pepsin and cathepsin D, respectively [1, 2].
Pepstatin A is a pentapeptide. It was originally isolated from the microbe. As a most potent renin inhibitor, pepstatinA inhibited porcine renin and human renin at weak acid pH value with IC50 values of 0.32 and 15 μM, respectively. The disadvantage is that, pepstatin Ais hydrophobic. To couple the charged hydrophilic residues to the C-terminal of pepstatinA can increase its solubility. Besides the renin, pepstatin A was also reported to have inhibitory effects on HIV protease and subsequently suppressed the virus replication. In cultured H9 cells, pepstatinA treatment blocked the proteolytic processing of the virus gag precursor and inhibited the production of infectious HIV. Moreover, pepstatin A was found to inhibit osteoclast differentiation due to its inhibitory efficacy of cathepsin D and E [1, 3 and 4].
References:
1. Eid M, Evin G, Castro B, et al.New renin inhibitors homologous with pepstatin.Biochem. J, 1981, 197: 465-471.
2. Sarubbi E, Seneci P F, Angelastro M R, et al. Peptide aldehydes as inhibitors of HIV protease. FEBS letters, 1993, 319(3): 253-256.
3. von der Helm K, Gürtler L, Eberle J, et al. Inhibition of HIV replication in cell culture by the specific aspartic protease inhibitor pepstatin A. FEBS letters, 1989, 247(2): 349-352.
4. Yoshida H, Okamoto K, Iwamoto T, et al. Pepstatin A, an aspartic proteinase inhibitor, suppresses RANKL-induced osteoclast differentiation. Journal of biochemistry, 2006, 139(3): 583-590. >
- Physcion-8-O-beta-D-monoglucoside
Catalog No.:BCN8511
CAS No.:26296-54-8
- Ficaprenol 11
Catalog No.:BCN5140
CAS No.:26296-50-4
- 2,3',4,6-Tetrahydroxybenzophenone
Catalog No.:BCN5139
CAS No.:26271-33-0
- AICAR
Catalog No.:BCC3606
CAS No.:2627-69-2
- Peritassine A
Catalog No.:BCC9117
CAS No.:262601-67-2
- Boc-β-Homo-Pro-OH
Catalog No.:BCC2628
CAS No.:26250-84-0
- BVD 10
Catalog No.:BCC5882
CAS No.:262418-00-8
- Cyclofenil
Catalog No.:BCC7839
CAS No.:2624-43-3
- 7,15-Dihydroxypodocarp-8(14)-en-13-one
Catalog No.:BCN1470
CAS No.:262355-96-4
- Torcetrapib
Catalog No.:BCC2330
CAS No.:262352-17-0
- Mudanpioside J
Catalog No.:BCC9050
CAS No.:262350-52-7
- Debenzoylgalloylpaeoniflorin
Catalog No.:BCC8927
CAS No.:262350-51-6
- 22-Dehydroclerosterol
Catalog No.:BCN5141
CAS No.:26315-07-1
- Dehydroperilloxin
Catalog No.:BCN7506
CAS No.:263241-09-4
- Perilloxin
Catalog No.:BCN6614
CAS No.:263249-77-0
- (S)-(-)-Pindolol
Catalog No.:BCC6916
CAS No.:26328-11-0
- 4-(trans)-Acetyl-3,6,8-trihydroxy-3-methyldihydronaphthalenone
Catalog No.:BCN1469
CAS No.:263368-91-8
- 4-(cis)-Acetyl-3,6,8-trihydroxy-3-methyldihydronaphthalenone
Catalog No.:BCN1468
CAS No.:263368-92-9
- Escin IB
Catalog No.:BCN2970
CAS No.:26339-90-2
- Aesculuside B
Catalog No.:BCC8115
CAS No.:26339-92-4
- 1,2-Benzisothiazolin-3-one
Catalog No.:BCC8412
CAS No.:2634-33-5
- H-Arg-OMe.2HCl
Catalog No.:BCC2861
CAS No.:26340-89-6
- Rhodojaponin III
Catalog No.:BCN2809
CAS No.:26342-66-5
- H-Lys-OMe .2HCl
Catalog No.:BCC2981
CAS No.:26348-70-9
Design, synthesis, inhibition studies, and molecular modeling of pepstatin analogues addressing different secreted aspartic proteinases of Candida albicans.[Pubmed:23262278]
Biochem Pharmacol. 2013 Apr 1;85(7):881-7.
The family of secreted aspartic proteinases is known as an important virulence factor of yeast infections by Candida albicans in particular, which is the most common fungal pathogen for humans with respect to systemic disease. Due to the continuing increase of drug resistant strains, these proteinases are currently considered as promising drug target candidates. Based on the known Sap2-substrate specificity data and X-ray analyses of Sap/inhibitor complexes, three libraries of inhibitors were designed and synthesized by modifying the structure of Pepstatin A, a common non-selective aspartic proteinase inhibitor, at the P3, P2, or P2' position. These novel inhibitors showed high inhibitory potencies for the isoenzymes Sap1, Sap3, Sap5 and Sap6. Then, the affinity and selectivity of the peptide ligands were investigated by molecular modeling, highlighting new key structural information for the design of potent and selective anti-virulence agents targeting Candida albicans.
A multimodal Pepstatin A peptide-based nanoagent for the molecular imaging of P-glycoprotein in the brains of epilepsy rats.[Pubmed:26524537]
Biomaterials. 2016 Jan;76:173-86.
Regional overexpression of the multidrug transporter P-glycoprotein (P-gp) in epileptic brain tissues may lower antiepileptic drugs concentrations at the target site and contribute to pharmacoresistance in refractory epilepsy. However, few techniques are available to quantitate the level of P-gp expression noninvasively in vivo. In this study, we developed a nanoagent by conjugating superparamagnetic iron oxide nanoparticles with a near infrared probe and the targeting element Pepstatin A, a peptide with specific affinity for P-gp. In a rat model of epilepsy, the nanoagent was readily and selectively accumulated within epileptogenic cerebral regions, which were detectable by both magnetic resonance imaging and optical imaging modalities. This P-gp-targeted nanoagent could be used not only in the molecular imaging of P-gp expression changes in seizure-induced regional, understanding the mechanisms of P-gp disorders, and the prediction of refractory epilepsy, but also in targeted therapies with P-gp modulators.
(1)H, (1)(3)C, and (1)(5)N backbone resonance assignments of the porcine pepsin and porcine pepsin complexed with pepstatin.[Pubmed:23264006]
Biomol NMR Assign. 2014 Apr;8(1):57-61.
Pepsin is formed as the zymogen, pepsinogen, which includes an additional 44 residue prosegment (PS) on the N-terminus. Upon acidification (pH <3) the PS is removed, yielding active pepsin. The PS is critical to such processes as the initiation of correct folding and protein stability. In the present study, the NMR assignments of the 34.6 kDa native porcine pepsin and porcine pepsin complexed with Pepstatin Are reported in order to obtain structural information regarding PS-catalyzed protein folding. Such information would contribute to a better understanding of the nature of folding/unfolding energy barrier of pepsin and other aspartic proteases.
A new pepstatin-insensitive thermopsin-like protease overproduced in peptide-rich cultures of Sulfolobus solfataricus.[Pubmed:24566144]
Int J Mol Sci. 2014 Feb 21;15(2):3204-19.
In this study, we gain insight into the extracellular proteolytic system of Sulfolobus solfataricus grown on proteinaceous substrates, providing further evidence that acidic proteases were specifically produced in response to peptide-rich media. The main proteolytic component was the previously isolated SsMTP (Sulfolobus solfataricus multi-domain thermopsin-like protease), while the less abundant (named SsMTP-1) one was purified, characterized and identified as the sso1175 gene-product. The protein revealed a multi-domain organization shared with the cognate SsMTP with a catalytic domain followed by several tandemly-repeated motifs. Moreover, both enzymes were found spread across the Crenarchaeota phylum and belonging to the thermopsin family, although segregated into diverse phylogenetic clusters. SsMTP-1 showed a 75-kDa molecular mass and was stable in the temperature range 50-90 degrees C, with optimal activity at 70 degrees C and pH 2.0. Serine, metallo and aspartic protease inhibitors did not affect the enzyme activity, designating SsMTP-1 as a new member of the pepstatin-insensitive aspartic protease family. The peptide-bond-specificity of SsMTP-1 in the cleavage of the oxidized insulin B chain was uncommon amongst thermopsins, suggesting that it could play a distinct, but cooperative role in the protein degradation machinery. Interestingly, predictions of the transmembrane protein topology of SsMTP and SsMTP-1 strongly suggest a possible contribution in signal-transduction pathways.
Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation.[Pubmed:17942897]
Cancer Res. 2007 Oct 15;67(20):9677-84.
Several types of cancer cells, including colorectal cancer-derived cell lines, show austerity, the resistance to nutrient starvation, but exactly how cancer cells obtain energy sources under conditions in which their external nutrient supply is extremely limited remains to be clarified. Because autophagy is a catabolic process by which cells supply amino acids from self-digested organelles, cancer cells are likely to use autophagy to obtain amino acids as alternative energy sources. Amino acid deprivation-induced autophagy was assessed in DLD-1 and other colorectal cancer-derived cell lines. The autophagosome-incorporated LC3-II protein level increased after treatment with a combination of autolysosome inhibitors, which interferes with the consumption of autophagosomes. Autophagosome formation was also morphologically confirmed using ectopically expressed green fluorescent protein-LC3 fusion proteins in DLD-1 and SW480 cells. These data suggest that autophagosomes were actively produced and promptly consumed in colorectal cancer cells under nutrient starvation. Autolysosome inhibitors and 3-methyl adenine, which suppresses autophagosome formation, remarkably enhanced apoptosis under amino acid-deprived and glucose-deprived condition. Similar results were obtained in the cells with decreased ATG7 level by the RNA interference. These data suggest that autophagy is pivotal for the survival of colorectal cancer cells that have acquired austerity. Furthermore, autophagosome formation was seen only in the tumor cells but not in the adjacent noncancerous epithelial cells of colorectal cancer specimens. Taken together, autophagy is activated in colorectal cancers in vitro and in vivo, and autophagy may contribute to the survival of the cancer cells in their microenvironment.
Mode of inhibition of acid proteases by pepstatin.[Pubmed:993206]
J Biol Chem. 1976 Nov 25;251(22):7088-94.
Four derivatives of pepstatin, each of which contains the unusual amino acid 4-amino-3-hydroxy-6-methylheptanoic acid (statine) have been prepared. All four are porcine pepsin inhibitors. Both N-acetylstatine and N-acetyl-alanyl-statine are competitive inhibitors for pepsin with Ki values of 1.2 X 10(-4) M and 5.65 X 10(-6) M, respectively. The Ki values for N-acetyl-valyl-statine is 4.8 X 10(-6) M. These statyl derivatives, therefore, are very strong inhibitors. The Ki value for N-acetyl-statine is 600-fold smaller than that of its structural analog N-acetyl-leucine. The derivative which contains two statyl residues in a tetrapeptide exhibits inhibitory properties which approach those of pepstatin itself. Other acid proteases, human pepsin, human gastricsin, renin, cathepsin D, the acid protease from Rhizopus chinensis and bovine chymosin, also are inhibited by Pepstatin And its derivatives. It is suggested that the statyl residue is responsible for the unusual inhibitory capability of Pepstatin And that statine is an analog of the previously proposed transition state for catalysis by pepsin and other acid proteases.


