BVD 10NPY Y1 receptor antagonist,highly selective CAS# 262418-00-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 262418-00-8 | SDF | Download SDF |
| PubChem ID | 10820196 | Appearance | Powder |
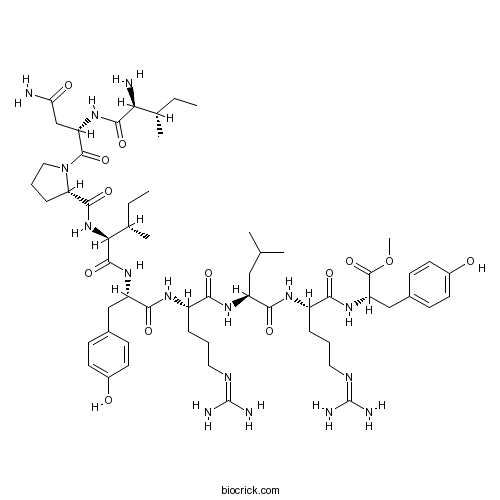
| Formula | C58H92N16O13 | M.Wt | 1221.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
| Sequence | INPIYRLRY (Modifications: Tyr-9 = Tyr-OMe) | ||
| Chemical Name | methyl (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3S)-2-[[(2S)-1-[(2S)-4-amino-2-[[(2S,3S)-2-amino-3-methylpentanoyl]amino]-4-oxobutanoyl]pyrrolidine-2-carbonyl]amino]-3-methylpentanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-4-methylpentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-3-(4-hydroxyphenyl)propanoate | ||
| SMILES | CCC(C)C(C(=O)NC(CC(=O)N)C(=O)N1CCCC1C(=O)NC(C(C)CC)C(=O)NC(CC2=CC=C(C=C2)O)C(=O)NC(CCCN=C(N)N)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC(CC3=CC=C(C=C3)O)C(=O)OC)N | ||
| Standard InChIKey | JLQHEFJKMUJISI-QUCBMPEKSA-N | ||
| Standard InChI | InChI=1S/C58H92N16O13/c1-8-32(5)46(60)53(83)71-42(30-45(59)77)55(85)74-26-12-15-44(74)52(82)73-47(33(6)9-2)54(84)70-41(28-34-16-20-36(75)21-17-34)51(81)68-38(13-10-24-65-57(61)62)48(78)69-40(27-31(3)4)50(80)67-39(14-11-25-66-58(63)64)49(79)72-43(56(86)87-7)29-35-18-22-37(76)23-19-35/h16-23,31-33,38-44,46-47,75-76H,8-15,24-30,60H2,1-7H3,(H2,59,77)(H,67,80)(H,68,81)(H,69,78)(H,70,84)(H,71,83)(H,72,79)(H,73,82)(H4,61,62,65)(H4,63,64,66)/t32-,33-,38-,39-,40-,41-,42-,43-,44-,46-,47-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly selective NPY Y1 receptor antagonist. Ki values are 25.7, 1420, 2403 and 7100 nM at Y1, Y2, Y4 and Y5 receptors respectively. Devoid of agonist activity at Y4 receptors. |

BVD 10 Dilution Calculator

BVD 10 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ki: 25.7 nM for Y1
Neuropeptide Y (NPY), a 36-residue peptide amide isolated originally from porcine brain, is a member of homologous hormone family including peptide YY (PYY) and pancreatic polypeptide (PP). It is the most abundant peptide in the mammalian brain and has been shown to exhibit a wide spectrum of central and peripheral activities. These actions are mediated by at least six G-protein coupled receptor subtypes denoted as Y1, Y2, Y3, Y4, Y5, and y6. BVD 10 is a highly selective and potent neuropeptide Y (NPY) Y1 receptor antagonists.
In vitro: The inability to form such hydrogen bonding in BVD 10 may prevent or perturb the C-terminus reverse turn, which may contribute, at least in part, to the increased Y1 selectivity [1]. Moreover, BVD 10 and its NPY analogue peptide BVD15 were characterized conformationally. The two peptides exhibit different secondary structure characteristics in trifluoroethanol. Molecular modeling studies suggested that the C-terminus Tyr9 is oriented in different directions in the two peptides. The difference in the structures observed may contribute to the Y1 selectivity of BVD 10 relative to BVD 15 [2].
In vivo: No animal in vivo data have been reported so far.
Clinical trial: Up to now, BVD 10 is still in the preclinical development stage.
References:
[1] Balasubramaniam A, Dhawan VC, Mullins DE, Chance WT, Sheriff S, Guzzi M, Prabhakaran M, Parker EM. Highly selective and potent neuropeptide Y (NPY) Y1 receptor antagonists based on [Pro(30), Tyr(32), Leu(34)]NPY(28-36)-NH2 (BW1911U90). J Med Chem. 2001 May 10;44(10):1479-82.
[2] Jois SD, Balasubramaniam A. Conformation of neuropeptide Y receptor antagonists: structural implications in receptor selectivity. Peptides. 2003 Jul;24(7):1035-43.
- Cyclofenil
Catalog No.:BCC7839
CAS No.:2624-43-3
- 7,15-Dihydroxypodocarp-8(14)-en-13-one
Catalog No.:BCN1470
CAS No.:262355-96-4
- Torcetrapib
Catalog No.:BCC2330
CAS No.:262352-17-0
- Mudanpioside J
Catalog No.:BCC9050
CAS No.:262350-52-7
- Debenzoylgalloylpaeoniflorin
Catalog No.:BCC8927
CAS No.:262350-51-6
- H-D-Aib-OH
Catalog No.:BCC3151
CAS No.:2623-91-8
- 3,6-Dihydroxy-1,7-dimethoxyxanthone
Catalog No.:BCN6394
CAS No.:262292-34-2
- 3alpha-Acetoxy-20-oxo-29-norlupane-23,28-dioic acid
Catalog No.:BCN6507
CAS No.:262272-76-4
- Cl-HOBt
Catalog No.:BCC2829
CAS No.:26198-19-6
- CALP3
Catalog No.:BCC5900
CAS No.:261969-05-5
- CALP2
Catalog No.:BCC5898
CAS No.:261969-04-4
- Isotaxiresinol
Catalog No.:BCN4660
CAS No.:26194-57-0
- Boc-β-Homo-Pro-OH
Catalog No.:BCC2628
CAS No.:26250-84-0
- Peritassine A
Catalog No.:BCC9117
CAS No.:262601-67-2
- AICAR
Catalog No.:BCC3606
CAS No.:2627-69-2
- 2,3',4,6-Tetrahydroxybenzophenone
Catalog No.:BCN5139
CAS No.:26271-33-0
- Ficaprenol 11
Catalog No.:BCN5140
CAS No.:26296-50-4
- Physcion-8-O-beta-D-monoglucoside
Catalog No.:BCN8511
CAS No.:26296-54-8
- Pepstatin A
Catalog No.:BCC1218
CAS No.:26305-03-3
- 22-Dehydroclerosterol
Catalog No.:BCN5141
CAS No.:26315-07-1
- Dehydroperilloxin
Catalog No.:BCN7506
CAS No.:263241-09-4
- Perilloxin
Catalog No.:BCN6614
CAS No.:263249-77-0
- (S)-(-)-Pindolol
Catalog No.:BCC6916
CAS No.:26328-11-0
- 4-(trans)-Acetyl-3,6,8-trihydroxy-3-methyldihydronaphthalenone
Catalog No.:BCN1469
CAS No.:263368-91-8
Modeling of neuropeptide receptors Y1, Y4, Y5, and docking studies with neuropeptide antagonist.[Pubmed:16494499]
J Biomol Struct Dyn. 2006 Apr;23(5):497-508.
Neuropeptide Y (NPY), receptors belong to the G-protein coupled receptor superfamily. NPY mediates several physiological responses, such as blood pressure, food intake, sedation. These actions of NPY are mediated by six receptor subtypes denoted as Y1-Y5 and y6. Modeling of receptor subtypes and binding site identification is an important step in developing new therapeutic agents. We have attempted to model the three NPY receptor types, Y1, Y4, and Y5 using homology modeling and threading methods. The models are consistent with previously reported experimental evidence. To understand the interaction and selectivity of NPY analogues with different neuropeptide receptors, docking studies of two neuropeptide analogues (BVD10 and BVD15) with receptors Y1 and Y4 were carried out. Results of the docking studies indicated that the interaction of ligands BVD10 and BVD15 with Y1 and Y4 receptors are different. These results were evaluated for selectivity of peptide analogues BVD10 and BVD15 towards the receptors.
Conformation of neuropeptide Y receptor antagonists: structural implications in receptor selectivity.[Pubmed:14499282]
Peptides. 2003 Jul;24(7):1035-43.
Two NPY analogue peptides, BVD10 (Ile-Asn-Pro-Ile-Tyr-Arg-Leu-Arg-Tyr-OMe) and BVD15 (Ile-Asn-Pro-Ile-Tyr-Arg-Leu-Arg-Tyr-NH(2)) were characterized conformationally by NMR, CD and molecular dynamics simulations. The two peptides exhibit different secondary structure characteristics in trifluoroethanol. BVD10 exhibits a structure with two consecutive beta-turns at Asn2-Pro3-Ile4-Tyr5 and Ile4-Tyr5-Arg6-Leu7. BVD15 exhibits a helical type of structure along with a beta-turn at Asn2-Pro3-Ile4-Tyr5. Molecular modeling studies suggested that the C-terminus Tyr9 is oriented in different directions in the two peptides. The difference in the structures of peptides observed may contribute to the Y(1) selectivity of BVD10 relative to BVD15.


