Peritassine ACAS# 262601-67-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 262601-67-2 | SDF | Download SDF |
| PubChem ID | 131676056 | Appearance | Powder |
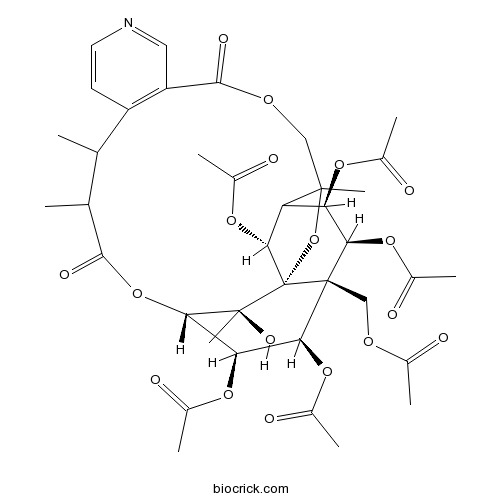
| Formula | C38H47NO18 | M.Wt | 805.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1C(C(=O)OC2C(C(C3(C(C(C4C(C3(C2(C)O)OC4(COC(=O)C5=C1C=CN=C5)C)OC(=O)C)OC(=O)C)OC(=O)C)COC(=O)C)OC(=O)C)OC(=O)C)C | ||
| Standard InChIKey | XVCIECFQBMGYAF-MNNNDVGPSA-N | ||
| Standard InChI | InChI=1S/C38H47NO18/c1-16-17(2)33(46)56-30-28(52-20(5)42)32(55-23(8)45)37(15-49-18(3)40)31(54-22(7)44)27(51-19(4)41)26-29(53-21(6)43)38(37,36(30,10)48)57-35(26,9)14-50-34(47)25-13-39-12-11-24(16)25/h11-13,16-17,26-32,48H,14-15H2,1-10H3/t16?,17?,26?,27-,28+,29-,30+,31-,32+,35?,36+,37-,38+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Peritassine A Dilution Calculator

Peritassine A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.241 mL | 6.205 mL | 12.41 mL | 24.8201 mL | 31.0251 mL |
| 5 mM | 0.2482 mL | 1.241 mL | 2.482 mL | 4.964 mL | 6.205 mL |
| 10 mM | 0.1241 mL | 0.6205 mL | 1.241 mL | 2.482 mL | 3.1025 mL |
| 50 mM | 0.0248 mL | 0.1241 mL | 0.2482 mL | 0.4964 mL | 0.6205 mL |
| 100 mM | 0.0124 mL | 0.0621 mL | 0.1241 mL | 0.2482 mL | 0.3103 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boc-β-Homo-Pro-OH
Catalog No.:BCC2628
CAS No.:26250-84-0
- BVD 10
Catalog No.:BCC5882
CAS No.:262418-00-8
- Cyclofenil
Catalog No.:BCC7839
CAS No.:2624-43-3
- 7,15-Dihydroxypodocarp-8(14)-en-13-one
Catalog No.:BCN1470
CAS No.:262355-96-4
- Torcetrapib
Catalog No.:BCC2330
CAS No.:262352-17-0
- Mudanpioside J
Catalog No.:BCC9050
CAS No.:262350-52-7
- Debenzoylgalloylpaeoniflorin
Catalog No.:BCC8927
CAS No.:262350-51-6
- H-D-Aib-OH
Catalog No.:BCC3151
CAS No.:2623-91-8
- 3,6-Dihydroxy-1,7-dimethoxyxanthone
Catalog No.:BCN6394
CAS No.:262292-34-2
- 3alpha-Acetoxy-20-oxo-29-norlupane-23,28-dioic acid
Catalog No.:BCN6507
CAS No.:262272-76-4
- Cl-HOBt
Catalog No.:BCC2829
CAS No.:26198-19-6
- CALP3
Catalog No.:BCC5900
CAS No.:261969-05-5
- AICAR
Catalog No.:BCC3606
CAS No.:2627-69-2
- 2,3',4,6-Tetrahydroxybenzophenone
Catalog No.:BCN5139
CAS No.:26271-33-0
- Ficaprenol 11
Catalog No.:BCN5140
CAS No.:26296-50-4
- Physcion-8-O-beta-D-monoglucoside
Catalog No.:BCN8511
CAS No.:26296-54-8
- Pepstatin A
Catalog No.:BCC1218
CAS No.:26305-03-3
- 22-Dehydroclerosterol
Catalog No.:BCN5141
CAS No.:26315-07-1
- Dehydroperilloxin
Catalog No.:BCN7506
CAS No.:263241-09-4
- Perilloxin
Catalog No.:BCN6614
CAS No.:263249-77-0
- (S)-(-)-Pindolol
Catalog No.:BCC6916
CAS No.:26328-11-0
- 4-(trans)-Acetyl-3,6,8-trihydroxy-3-methyldihydronaphthalenone
Catalog No.:BCN1469
CAS No.:263368-91-8
- 4-(cis)-Acetyl-3,6,8-trihydroxy-3-methyldihydronaphthalenone
Catalog No.:BCN1468
CAS No.:263368-92-9
- Escin IB
Catalog No.:BCN2970
CAS No.:26339-90-2
[Study on chemical constituents from the root bark of Tripterygium hypoglaucum].[Pubmed:23252270]
Zhong Yao Cai. 2012 Jul;35(7):1083-7.
OBJECTIVE: To study the chemical constituents of the root bark of Tripterygium hypoglaucum. METHODS: Column chromatography was used to separate the chemical constituents. The structures were determined by application of spectroscopic (NMR, MS) and chemical methods. RESULTS: Eleven compounds were isolated and identified as 4'-0-(-) methylepigallocatechin (1),3,4-dimethoxyphenyl-beta-D-glucopyranoside (2), 3,4,5-trimethoxyphenyl-f-D-glucopyranoside (3), (2R,3R)-3,5,7,3',5'-pentahydroxyflavan (4), 2-O-deacetyleuonine (5), tripfordine C (6), Peritassine A (7) hypoglaunine C (8), wilfortrine (9), wilforgine (10) and wilfordine (11). CONCLUSION: Compounds 1-6 are isolated from this plant for the first time.
Preparative separation of a terpenoid and alkaloids from Tripterygium wilfordii Hook. f. using high-performance counter-current chromatography. Comparison of various elution and operating strategies.[Pubmed:18976997]
J Chromatogr A. 2008 Dec 12;1213(2):145-53.
This paper describes how high-performance counter-current chromatography (HPCCC) was used strategically for the separation of Tripterygium wilfordii Hook. f. Due to the complexity of Chinese herbal medicines, the initial ethanol crude extract was fractionated into seven fractions using medium-pressure liquid chromatography (MPLC). One terpenoid (triptolide) and three alkaloids (Peritassine A, wilforgine and wilforine) were further separated from one of the MPLC fractions. This fraction (1.25 g) yielded 8 mg of triptolide and 28 mg of peritassines A after one HPCCC column pass and 30 mg of wilforgine and 120 mg of wilforine after a second column pass with respective purities of 97%, 93.6%, 95.0% and 94.4%, which were determined by high-performance liquid chromatography (HPLC). This was a one-step HPCCC separation, using an n-hexane-ethyl acetate-methanol-water (4:5:4:5, v/v) solvent system, where increases in theoretical plates have been sacrificed in favour of increasing throughput. Structures were identified by electrospray ionization mass spectrometry (ESI-MS), (1)H nuclear magnetic resonance ((1)H NMR) and (13)C nuclear magnetic resonance ((13)C NMR). Comparison of three different modes of eluting compounds retained in the liquid stationary phase: elution extrusion; dual mode and simple pump-out showed that simply pumping out the column contents at high flow gave better resolution and was eight times faster than the other two well-utilised methods. Triptolide and peritassines A were isolated for the first time from Tripterygium wilfordii Hook. f.
Sesquiterpene alkaloids from Tripterygium hypoglaucum and Tripterygium wilfordii: a new class of potent anti-HIV agents.[Pubmed:10757718]
J Nat Prod. 2000 Mar;63(3):357-61.
Five new sesquiterpene pyridine alkaloids [triptonines A (1) and B (2), and wilfordinines A (3), B (4), and C (5)] and two known compounds (Peritassine A and hypoglaunine C) were isolated from Tripterygium hypoglaucum and a clinically used extract of Tripterygium wilfordii. The structures of 1-5 were elucidated by spectroscopic methods. The anti-HIV activity of 1, 2, and several related compounds was evaluated. Triptonine B (2) demonstrated potent anti-HIV activity with an EC(50) value of <0.10 microg/mL and an in vitro therapeutic index value of >1000.


