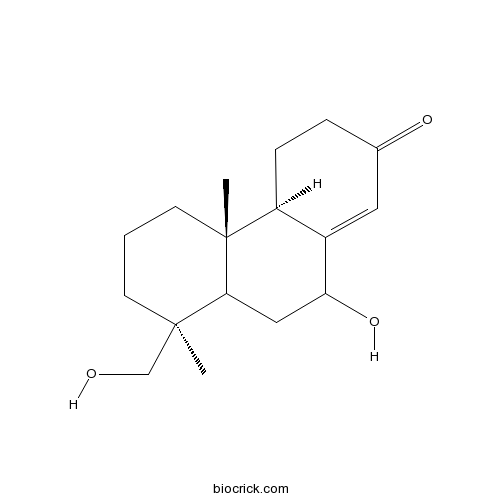
7,15-Dihydroxypodocarp-8(14)-en-13-oneCAS# 262355-96-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 262355-96-4 | SDF | Download SDF |
| PubChem ID | 71307291 | Appearance | Powder |
| Formula | C17H26O3 | M.Wt | 278.4 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4aR,4bS,8S)-10-hydroxy-8-(hydroxymethyl)-4b,8-dimethyl-4,4a,5,6,7,8a,9,10-octahydro-3H-phenanthren-2-one | ||
| SMILES | CC1(CCCC2(C1CC(C3=CC(=O)CCC32)O)C)CO | ||
| Standard InChIKey | ZAYXCFZRTAJXMC-LMCYLSQRSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

7,15-Dihydroxypodocarp-8(14)-en-13-one Dilution Calculator

7,15-Dihydroxypodocarp-8(14)-en-13-one Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.592 mL | 17.9598 mL | 35.9195 mL | 71.8391 mL | 89.7989 mL |
| 5 mM | 0.7184 mL | 3.592 mL | 7.1839 mL | 14.3678 mL | 17.9598 mL |
| 10 mM | 0.3592 mL | 1.796 mL | 3.592 mL | 7.1839 mL | 8.9799 mL |
| 50 mM | 0.0718 mL | 0.3592 mL | 0.7184 mL | 1.4368 mL | 1.796 mL |
| 100 mM | 0.0359 mL | 0.1796 mL | 0.3592 mL | 0.7184 mL | 0.898 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Torcetrapib
Catalog No.:BCC2330
CAS No.:262352-17-0
- Mudanpioside J
Catalog No.:BCC9050
CAS No.:262350-52-7
- Debenzoylgalloylpaeoniflorin
Catalog No.:BCC8927
CAS No.:262350-51-6
- H-D-Aib-OH
Catalog No.:BCC3151
CAS No.:2623-91-8
- 3,6-Dihydroxy-1,7-dimethoxyxanthone
Catalog No.:BCN6394
CAS No.:262292-34-2
- 3alpha-Acetoxy-20-oxo-29-norlupane-23,28-dioic acid
Catalog No.:BCN6507
CAS No.:262272-76-4
- Cl-HOBt
Catalog No.:BCC2829
CAS No.:26198-19-6
- CALP3
Catalog No.:BCC5900
CAS No.:261969-05-5
- CALP2
Catalog No.:BCC5898
CAS No.:261969-04-4
- Isotaxiresinol
Catalog No.:BCN4660
CAS No.:26194-57-0
- SB269970 HCl
Catalog No.:BCC5056
CAS No.:261901-57-9
- Foliamenthoic acid
Catalog No.:BCN5138
CAS No.:26187-80-4
- Cyclofenil
Catalog No.:BCC7839
CAS No.:2624-43-3
- BVD 10
Catalog No.:BCC5882
CAS No.:262418-00-8
- Boc-β-Homo-Pro-OH
Catalog No.:BCC2628
CAS No.:26250-84-0
- Peritassine A
Catalog No.:BCC9117
CAS No.:262601-67-2
- AICAR
Catalog No.:BCC3606
CAS No.:2627-69-2
- 2,3',4,6-Tetrahydroxybenzophenone
Catalog No.:BCN5139
CAS No.:26271-33-0
- Ficaprenol 11
Catalog No.:BCN5140
CAS No.:26296-50-4
- Physcion-8-O-beta-D-monoglucoside
Catalog No.:BCN8511
CAS No.:26296-54-8
- Pepstatin A
Catalog No.:BCC1218
CAS No.:26305-03-3
- 22-Dehydroclerosterol
Catalog No.:BCN5141
CAS No.:26315-07-1
- Dehydroperilloxin
Catalog No.:BCN7506
CAS No.:263241-09-4
- Perilloxin
Catalog No.:BCN6614
CAS No.:263249-77-0
Crystal structure of (1S,2R,4R,9S,11S,12R)-9alpha-hy-droxy-4,8-dimethyl-12-[(thio-morpholin-4-yl)meth- yl]-3,14-dioxatri-cyclo-[9.3.0.0(2,4)]tetra-dec-7-en-13-one.[Pubmed:25878873]
Acta Crystallogr E Crystallogr Commun. 2015 Jan 31;71(Pt 2):o140-1.
The title compound, C19H29NO4S, was synthesised from 9alpha-hy-droxy-parthenolide (9alpha-hy-droxy-4,8-dimethyl-12-methyl-ene-3,14-dioxatri-cyclo-[9.3.0.0(2,4)]tet ra-dec-7-en-13-one), which was isolated from the chloro-form extract of the aerial parts of the plant Anvillea radiata. The mol-ecule is built up from two fused five- and ten-membered rings, with an additional ep-oxy ring system and a thio-morpholine group as a substituent. The ten-membered ring adopts an approximate chair-chair conformation, while the thio-morpholine ring displays a chair conformation and the five-membered ring has an envelope conformation, with the C atom closest to the hy-droxy group forming the flap. An intra-molecular O-Hcdots, three dots, centeredN hydrogen bond closes an S(8) ring. The crystal structure features weak C-Hcdots, three dots, centeredO hydrogen-bonding inter-actions, which link the mol-ecules into [010] chains.
12-{[4-(2-Fluoro-phen-yl)piperazin-1-yl]-meth-yl}-9alpha-hy-droxy-4,8-dimethyl-3, 14-dioxatricyclo-[9.3.0.0]tetra-dec-7-en-13-one.[Pubmed:22412621]
Acta Crystallogr Sect E Struct Rep Online. 2012 Mar 1;68(Pt 3):o741-2.
The title compound, C(25)H(33)FN(2)O(4), was synthesized from 9alpha-hy-droxy-parthenolide (9alpha-hy-droxy-4,8-dimethyl-12-methyl-ene-3,14-dioxatricyclo-[9.3.0.0(2,4)]tetr a-dec-7-en-13-one), which was isolated from the chloro-form extract of the aerial parts of Anvillea radiata. The asymmetric unit contains two independent mol-ecules. In each mol-ecule, the ten-membered ring displays an approximative chair-chair conformation. Each of the piperazine rings adopts a perfect chair conformation, while both lactone rings show an envelope conformation, one with the C atom bearing the piperazin-1-ylmethyl group as the flap, the other with the junction C atom not attached to the ring O atom as the flap. The dihedral angles between the least-squares planes through the ten- and five-membered rings in the two mol-ecules are similar [19.1 (3) and 16.2 (3) degrees ]. An intra-molecular O-Hcdots, three dots, centeredN hydrogen bond stabilizes the mol-ecular conformation. The crystal packing is stabilized by C-Hcdots, three dots, centeredO hydrogen bonds.
9alpha-Hy-droxy-12-{[4-(4-hy-droxy-phen-yl)piperazin-1-yl]meth-yl}-4,8-dimethyl-3 ,14-dioxatri-cyclo-[9.3.0.0(2,4)]tetra-dec-7-en-13-one.[Pubmed:24860343]
Acta Crystallogr Sect E Struct Rep Online. 2014 Apr 9;70(Pt 5):o530-1.
The title compound, C25H34N2O5, was synthesized from 9alpha-hy-droxy-parthenolide (9alpha-hy-droxy-4,8-dimethyl-12-methylen-3, 14-dioxa-tri-cyclo-[9.3.0.0(2,4)]tetra-dec-7-en-13-one), which in turn was isolated from the chloro-form extract of the aerial parts of Anvillea radiata. The mol-ecule comprises a ten-membered ring fused to a five-membered ring with an additional ep-oxy ring system fused to the ten-membered ring. The five-membered ring also carries a 4-hy-droxy-phenyl-piperazin-1-ylmethyl substituent. The ten-membered ring adopts an approximate chair-chair conformation, while the piperazine ring displays a chair conformation and the five-membered ring shows an envelope conformation with the C atom closest to the hy-droxy group forming the flap. Two C atoms in the phenyl ring and the O atom of the hydroxyl group are disordered over two sites, with an occupancy ratio of 0.53 (5):0.47 (5). An intra-molecular O-Hcdots, three dots, centeredN hydrogen-bond stabilizes the mol-ecular conformation. In the crystal, C-Hcdots, three dots, centeredO hydrogen bonds link the mol-ecules into zigzag chains running along the a-axis direction.
12-{[4-(4-Bromo-phen-yl)piperazin-1-yl]meth-yl}-9alpha-hy-droxy-4,8-dimethyl-3,14 -dioxatri-cyclo-[9.3.0.0(2,4)]tetra-dec-7-en-13-one.[Pubmed:24826186]
Acta Crystallogr Sect E Struct Rep Online. 2014 Mar 29;70(Pt 4):o497-8.
The title compound, C25H33BrN2O4, was synthesized from 9alpha-hy-droxy-parthenolide (9alpha-hy-droxy-4,8-dimethyl-12-methylen-3,14-dioxa-tri-cyclo-[9.3.0.0(2,4)]tetr a-dec-7-en-13-one), which was isolated from the chloro-form extract of the aerial parts of Anvillea radiata. The mol-ecule is built up from two fused five- and ten-membered rings with an additional ep-oxy ring system and a bromo-phenyl-piperazine group as a substituent. The ten-membered ring adopts an approximate chair-chair-chair conformation, while the piperazine ring displays a chair conformation and the five-membered ring shows an envelope conformation with the C atom closest to the hy-droxy group forming the flap. An intra-molecular O-Hcdots, three dots, centeredN hydrogen bond stabilizes the mol-ecular conformation. The crystal packing features C-Hcdots, three dots, centeredO hydrogen bonds, which link the mol-ecules into zigzag chains running along the b-axis direction.


