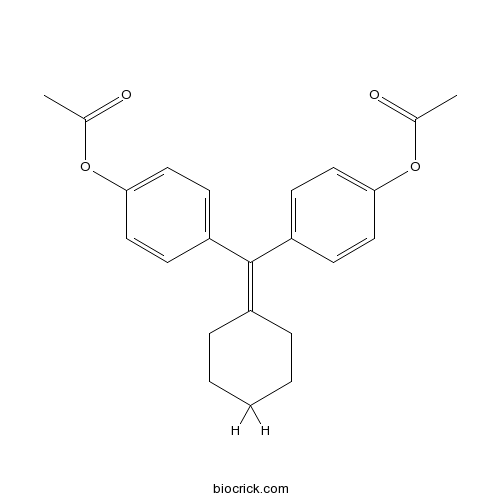
CyclofenilSelective estrogen receptor modulator (SERM) CAS# 2624-43-3 |
- MNS
Catalog No.:BCC3943
CAS No.:1485-00-3
- BIO 1211
Catalog No.:BCC3945
CAS No.:187735-94-0
- Cilengitide
Catalog No.:BCC3942
CAS No.:188968-51-6
- A 205804
Catalog No.:BCC3944
CAS No.:251992-66-2
- Firategrast
Catalog No.:BCC1575
CAS No.:402567-16-2
- Zaurategrast
Catalog No.:BCC2070
CAS No.:455264-31-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2624-43-3 | SDF | Download SDF |
| PubChem ID | 2898 | Appearance | Powder |
| Formula | C23H24O4 | M.Wt | 364.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in DMSO | ||
| Chemical Name | [4-[(4-acetyloxyphenyl)-cyclohexylidenemethyl]phenyl] acetate | ||
| SMILES | CC(=O)OC1=CC=C(C=C1)C(=C2CCCCC2)C3=CC=C(C=C3)OC(=O)C | ||
| Standard InChIKey | GVOUFPWUYJWQSK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H24O4/c1-16(24)26-21-12-8-19(9-13-21)23(18-6-4-3-5-7-18)20-10-14-22(15-11-20)27-17(2)25/h8-15H,3-7H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Non-steroidal selective estrogen receptor modulator (SERM). Displays higher affinity for estrogen receptor β (ERβ) than for ERα. |

Cyclofenil Dilution Calculator

Cyclofenil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.744 mL | 13.7201 mL | 27.4401 mL | 54.8802 mL | 68.6003 mL |
| 5 mM | 0.5488 mL | 2.744 mL | 5.488 mL | 10.976 mL | 13.7201 mL |
| 10 mM | 0.2744 mL | 1.372 mL | 2.744 mL | 5.488 mL | 6.86 mL |
| 50 mM | 0.0549 mL | 0.2744 mL | 0.5488 mL | 1.0976 mL | 1.372 mL |
| 100 mM | 0.0274 mL | 0.1372 mL | 0.2744 mL | 0.5488 mL | 0.686 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7,15-Dihydroxypodocarp-8(14)-en-13-one
Catalog No.:BCN1470
CAS No.:262355-96-4
- Torcetrapib
Catalog No.:BCC2330
CAS No.:262352-17-0
- Mudanpioside J
Catalog No.:BCC9050
CAS No.:262350-52-7
- Debenzoylgalloylpaeoniflorin
Catalog No.:BCC8927
CAS No.:262350-51-6
- H-D-Aib-OH
Catalog No.:BCC3151
CAS No.:2623-91-8
- 3,6-Dihydroxy-1,7-dimethoxyxanthone
Catalog No.:BCN6394
CAS No.:262292-34-2
- 3alpha-Acetoxy-20-oxo-29-norlupane-23,28-dioic acid
Catalog No.:BCN6507
CAS No.:262272-76-4
- Cl-HOBt
Catalog No.:BCC2829
CAS No.:26198-19-6
- CALP3
Catalog No.:BCC5900
CAS No.:261969-05-5
- CALP2
Catalog No.:BCC5898
CAS No.:261969-04-4
- Isotaxiresinol
Catalog No.:BCN4660
CAS No.:26194-57-0
- SB269970 HCl
Catalog No.:BCC5056
CAS No.:261901-57-9
- BVD 10
Catalog No.:BCC5882
CAS No.:262418-00-8
- Boc-β-Homo-Pro-OH
Catalog No.:BCC2628
CAS No.:26250-84-0
- Peritassine A
Catalog No.:BCC9117
CAS No.:262601-67-2
- AICAR
Catalog No.:BCC3606
CAS No.:2627-69-2
- 2,3',4,6-Tetrahydroxybenzophenone
Catalog No.:BCN5139
CAS No.:26271-33-0
- Ficaprenol 11
Catalog No.:BCN5140
CAS No.:26296-50-4
- Physcion-8-O-beta-D-monoglucoside
Catalog No.:BCN8511
CAS No.:26296-54-8
- Pepstatin A
Catalog No.:BCC1218
CAS No.:26305-03-3
- 22-Dehydroclerosterol
Catalog No.:BCN5141
CAS No.:26315-07-1
- Dehydroperilloxin
Catalog No.:BCN7506
CAS No.:263241-09-4
- Perilloxin
Catalog No.:BCN6614
CAS No.:263249-77-0
- (S)-(-)-Pindolol
Catalog No.:BCC6916
CAS No.:26328-11-0
Cyclofenil as a possible cause of non-arteritic anterior ischaemic optic neuropathy.[Pubmed:23264159]
BMJ Case Rep. 2012 Dec 20;2012. pii: bcr-2012-007825.
A 27-year-old woman receiving the steroid drug Cyclofenil as a fertility adjunct, experienced blurred vision 24 h after missing a dose and taking a double dose to 'catch up' with her therapeutic protocol. She was found to have a non-arteritic anterior ischaemic optic neuropathy with a visual hemifield defect and impaired optic nerve function, which has not since shown any recovery. This case highlights the prothrombotic potential for the drug when used above normal dosing range, and is therefore of great guidance for those initiating it as a fertility treatment, or in unlicensed use.
Fluorimetric determination of cyclofenil using photochemical derivatization.[Pubmed:18371802]
Talanta. 2008 Feb 15;74(5):1442-9.
In this work, a fluorimetric approach for the determination of Cyclofenil, 4-4'-(cyclohexylidenemethylene)bis(phenylacetate), is presented. The method was based on the intense fluorescence (250/410 nm) observed after a UV photochemical treatment of Cyclofenil. The influence of the pH and solvent system and UV exposure time on the fluorescence magnitude was studied. Optimization of parameters was made using experimental design (factorial design and central composite design). Limit of detection (3S(b)/m) were estimated to be 1.1 x 10(-8)mol L(-1) with the linear dynamic range extending up to 8 x 10(-5)mol L(-1). This analytical approach was tested through the analysis of a commercial pharmaceutical formulation. In this case, tests enabled an average recovery of 98.3+/-3.9% (for n=9) using the analytical curve. The identification of the fluorescent derivative is proposed based on results achieved from GC-MS.
Synthesis and identification of hydroxylated metabolites of the anti-estrogenic agent cyclofenil.[Pubmed:18576435]
J Mass Spectrom. 2008 Jul;43(7):958-64.
The detection of metabolites of the anti-estrogenic substance Cyclofenil, listed on the World Anti-Doping Agency (WADA) Prohibited List since 2004 is described. Target substances are hydroxylated metabolites, bearing an aliphatic hydroxyl group either in the 2-, 3- or 4-position of the aliphatic ring, in addition to the phenolic functions on the aromatic rings. Structural identification used NMR as well as high-resolution mass spectrometry after nano-electrospray ionisation (ESI). Unambiguous detection of all three synthesised Cyclofenil metabolites M1-M3 was done using gas chromatography for separation and electron ionisation mass spectrometry for detection of the per-silylated compounds in comparison with a reference urine deriving from an excretion study within the WADA 2007 Educational Programme.
Design, synthesis, and evaluation of cyclofenil derivatives for potential SPECT imaging agents.[Pubmed:20195693]
J Biol Inorg Chem. 2010 May;15(4):591-9.
To develop technetium- and rhenium-labeled nonsteroidal estrogen imaging agents for estrogen receptor (ER) positive breast tumors, two groups of rhenium and technetium Cyclofenil derivatives were synthesized and characterized. The binding affinities of the rhenium complexes for ERs were determined. The tricarbonyl rhenium complex showed the highest binding affinity for ERs (81.2 for ERbeta, 16.5 for ERalpha). Tricarbonyl technetium Cyclofenil complexes were obtained in high radiochemical purity and radiochemical yields. The results of studies of their octanol/water partition and in vitro stability are presented. These results demonstrate that these radiolabeled Cyclofenil derivatives may be considered as potential breast cancer imaging agents.
Characterization of the pharmacophore properties of novel selective estrogen receptor downregulators (SERDs).[Pubmed:20334372]
J Med Chem. 2010 Apr 22;53(8):3320-9.
Selective estrogen receptor (ER) down-regulators (SERDs) reduce ERalpha protein levels as well as block ER activity and therefore are promising therapeutic agents for the treatment of hormone refractory breast cancer. Starting with the triarylethylene acrylic acid SERD 4, we have investigated how alterations in both the ligand core structure and the appended acrylic acid substituent affect SERD activity. The new ligands were based on high affinity, symmetrical Cyclofenil or bicyclo[3.3.1]nonane core systems, and in these, the position of the carboxyl group was extended from the ligand core, either retaining the vinylic linkage of the substituent or replacing it with an ether linkage. Although most structural variants showed binding affinities for ERalpha and ERbeta higher than that of 4, only the compounds preserving the acrylic acid side chain retained SERD activity, although they could possess varying core structures. Hence, the acrylic acid moiety of the ligand is crucial for SERD-like blockade of ER activities.
Fluorine-substituted cyclofenil derivatives as estrogen receptor ligands: synthesis and structure-affinity relationship study of potential positron emission tomography agents for imaging estrogen receptors in breast cancer.[Pubmed:16610793]
J Med Chem. 2006 Apr 20;49(8):2496-511.
In a search for estrogen receptor (ER) ligands to be radiolabeled with fluorine-18 for imaging of ER-positive breast tumors with positron emission tomography (PET), we investigated Cyclofenil analogues substituted at the C3 or C4 position of the cyclohexyl group. McMurry coupling of 4,4'-dihydroxybenzophenone with various ketones produced key Cyclofenil intermediates, from which C3 and C4 substituents containing alkyl and various oxygen or fluorine-substituted alkyl groups were elaborated. Binding assays to both ERalpha and ERbeta revealed that the C3 site is more tolerant of steric bulk and polar groups than the C4 site, consistent with a computational model of the ERalpha ligand binding pocket. Fluorine substitution is tolerated very well at some sites, giving some compounds having affinities comparable to or higher than that of estradiol. These fluoro and fluoroalkyl Cyclofenils merit further consideration as fluorine-18 labeled ER ligands for PET imaging of ERs in breast tumors.


