AICARAMPK activator CAS# 2627-69-2 |
- SAMS Peptide
Catalog No.:BCC5745
CAS No.:125911-68-4
- YLF-466D
Catalog No.:BCC4086
CAS No.:1273323-67-3
- RSVA 405
Catalog No.:BCC8016
CAS No.:140405-36-3
- TCS-PIM-1-4a
Catalog No.:BCC5461
CAS No.:327033-36-3
- PT 1
Catalog No.:BCC7846
CAS No.:331002-70-1
- A-769662
Catalog No.:BCC2080
CAS No.:844499-71-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2627-69-2 | SDF | Download SDF |
| PubChem ID | 17513 | Appearance | Powder |
| Formula | C9H14N4O5 | M.Wt | 258.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Acadesine; AICA Riboside | ||
| Solubility | H2O : 65 mg/mL (251.71 mM; Need ultrasonic and warming) DMSO : ≥ 30 mg/mL (116.18 mM) *"≥" means soluble, but saturation unknown. | ||
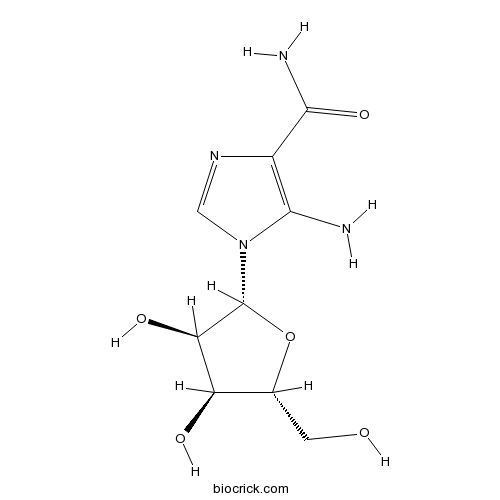
| Chemical Name | 5-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]imidazole-4-carboxamide | ||
| SMILES | C1=NC(=C(N1C2C(C(C(O2)CO)O)O)N)C(=O)N | ||
| Standard InChIKey | RTRQQBHATOEIAF-UUOKFMHZSA-N | ||
| Standard InChI | InChI=1S/C9H14N4O5/c10-7-4(8(11)17)12-2-13(7)9-6(16)5(15)3(1-14)18-9/h2-3,5-6,9,14-16H,1,10H2,(H2,11,17)/t3-,5-,6-,9-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cell-permeable, allosteric activator of AMP-activated protein kinase (AMPK). Augments proliferation, differentiation and mineralization of osteoblastic MC3T3-EI cells and attenuates psychosine-induced expression of proinflammatory cytokines and iNOS in astrocytes. Promotes osteogenic differentiation of hAMSCs and BM-MSCs in vitro. |

AICAR Dilution Calculator

AICAR Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8725 mL | 19.3626 mL | 38.7252 mL | 77.4503 mL | 96.8129 mL |
| 5 mM | 0.7745 mL | 3.8725 mL | 7.745 mL | 15.4901 mL | 19.3626 mL |
| 10 mM | 0.3873 mL | 1.9363 mL | 3.8725 mL | 7.745 mL | 9.6813 mL |
| 50 mM | 0.0775 mL | 0.3873 mL | 0.7745 mL | 1.549 mL | 1.9363 mL |
| 100 mM | 0.0387 mL | 0.1936 mL | 0.3873 mL | 0.7745 mL | 0.9681 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AICAR is a cell-permeable, allosteric activator of AMP-activated protein kinase (AMPK).
AMPK is a heterodimeric protein serine/threonine kinase that regulates the energy status of cells to protect cell from metabolic stress. AMPK phosphorylates various metabolic enzymes to activate catabolic pathways (e.g. ketogenesis) and block anabolic pathways (e.g. protein synthesis).
In rat primary astrocytes, microglia, and peritoneal macrophages, AICAR does-dependently inhibits the LPS-induced production of TNFα, IL-1β, and IL-6. AICAR treatment also blocks LPS-induced nitrite production and iNOS gene expression in those cells in a dose-dependent manner by activation of AMPK. Moreover, AICAR inhibits the LPS-induced C/EBP nuclear relocation via downregulating the expression of C/EBP-δ. [1]
In LPS-injected rats, AICAR treatment abolishes LPS-mediated increased levels of IL-1β and IFN-γ in serum. AICAR treatment also strongly inhibits the LPS-induced expression of iNOS in peritoneal macrophages isolated from these rats. Furthermore, the intraperitoneal injection of LPS significantly induces the expression of TNFα, IL-1β, and IFN-γ message in the rat spleen.
Reference:
1. Giri S, Nath N, Smith B et al. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci. 2004 Jan 14;24(2):479-87.
- Peritassine A
Catalog No.:BCC9117
CAS No.:262601-67-2
- Boc-β-Homo-Pro-OH
Catalog No.:BCC2628
CAS No.:26250-84-0
- BVD 10
Catalog No.:BCC5882
CAS No.:262418-00-8
- Cyclofenil
Catalog No.:BCC7839
CAS No.:2624-43-3
- 7,15-Dihydroxypodocarp-8(14)-en-13-one
Catalog No.:BCN1470
CAS No.:262355-96-4
- Torcetrapib
Catalog No.:BCC2330
CAS No.:262352-17-0
- Mudanpioside J
Catalog No.:BCC9050
CAS No.:262350-52-7
- Debenzoylgalloylpaeoniflorin
Catalog No.:BCC8927
CAS No.:262350-51-6
- H-D-Aib-OH
Catalog No.:BCC3151
CAS No.:2623-91-8
- 3,6-Dihydroxy-1,7-dimethoxyxanthone
Catalog No.:BCN6394
CAS No.:262292-34-2
- 3alpha-Acetoxy-20-oxo-29-norlupane-23,28-dioic acid
Catalog No.:BCN6507
CAS No.:262272-76-4
- Cl-HOBt
Catalog No.:BCC2829
CAS No.:26198-19-6
- 2,3',4,6-Tetrahydroxybenzophenone
Catalog No.:BCN5139
CAS No.:26271-33-0
- Ficaprenol 11
Catalog No.:BCN5140
CAS No.:26296-50-4
- Physcion-8-O-beta-D-monoglucoside
Catalog No.:BCN8511
CAS No.:26296-54-8
- Pepstatin A
Catalog No.:BCC1218
CAS No.:26305-03-3
- 22-Dehydroclerosterol
Catalog No.:BCN5141
CAS No.:26315-07-1
- Dehydroperilloxin
Catalog No.:BCN7506
CAS No.:263241-09-4
- Perilloxin
Catalog No.:BCN6614
CAS No.:263249-77-0
- (S)-(-)-Pindolol
Catalog No.:BCC6916
CAS No.:26328-11-0
- 4-(trans)-Acetyl-3,6,8-trihydroxy-3-methyldihydronaphthalenone
Catalog No.:BCN1469
CAS No.:263368-91-8
- 4-(cis)-Acetyl-3,6,8-trihydroxy-3-methyldihydronaphthalenone
Catalog No.:BCN1468
CAS No.:263368-92-9
- Escin IB
Catalog No.:BCN2970
CAS No.:26339-90-2
- Aesculuside B
Catalog No.:BCC8115
CAS No.:26339-92-4
AICAR induces mitochondrial apoptosis in human osteosarcoma cells through an AMPK-dependent pathway.[Pubmed:27878239]
Int J Oncol. 2017 Jan;50(1):23-30.
The AMP-activated protein kinase (AMPK) activator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) modulates cellular energy metabolism, and promotes mitochondrial proliferation and apoptosis. Previous studies have shown that AICAR has anticancer effects in various cancers, however the roles of AMPK and/or the effects of AICAR on osteosarcoma have not been reported. In the present study, we evaluated the effects of AICAR on tumor growth and mitochondrial apoptosis in human osteosarcoma both in vitro and in vivo. For in vitro experiments, two human osteosarcoma cell lines, MG63 and KHOS, were treated with AICAR, and the effects of AICAR on cell growth and mitochondrial apoptosis were assessed by WST assays, TUNEL staining, and immunoblot analyses. In vivo, human osteosarcoma-bearing mice were treated with AICAR, and the mitochondrial proliferation and apoptotic activity in treated tumors were assessed. In vitro experiments revealed that AICAR activated AMPK, inhibited cell growth, and induced mitochondrial apoptosis in both osteosarcoma cell lines. In vivo, AICAR significantly reduced osteosarcoma growth without apparent body weight loss and AICAR increased both mitochondrial proliferation and apoptotic activity in treated tumor tissues. AICAR showed anticancer effects in osteosarcoma cells through an AMPK-dependent peroxisome proliferatoractivated receptor-gamma coactivator-1alpha (PGC-1alpha)/mitochondrial transcription factor A (TFAM)/mitochondrial pathway. The findings in this study strongly suggest that AICAR could be considered as a potent therapeutic agent for the treatment of human osteosarcoma.
AICAR activates ER stress-dependent apoptosis in gallbladder cancer cells.[Pubmed:27847321]
Biochem Biophys Res Commun. 2017 Jan 8;482(2):246-252.
AICAR (5-Aminoimidazole-4-carboxamide riboside or acadesine) is an AMP-activated protein kinase (AMPK) agonist, its activity in human gallbladder cancer cells was evaluated here. We show that AICAR provoked significant apoptosis in human gallbladder cancer cell lines (Mz-ChA-1, QBC939 and GBC-SD) and primary gallbladder cancer cells. AICAR-induced cytotoxicity in gallbladder cancer cells appears independent of AMPK activation. Inhibition of AMPK, via AMPKalpha shRNA knockdown or dominant negative mutation (T172A), failed to rescue GBC-SD cells from AICAR. Further, forced-activation of AMPK, by adding two other AMPK activators (A769662 and Compound 13), or expressing a constitutively-active mutant AMPKalpha (T172D), didn't induce GBC-SD cell death. Remarkably, AICAR treatment in gallbladder cancer cells induced endoplasmic reticulum (ER) stress activation, the latter was tested by caspase-12 activation, C/EBP homologous protein (CHOP) expression and IRE1/PERK phosphorylation. Contrarily, salubrinal (the ER stress inhibitor), z-ATAD-fmk (the caspase-12 inhibitor) or CHOP shRNAs significantly attenuated AICAR-induced gallbladder cancer cell apoptosis. Together, we conclude that AICAR-induced gallbladder cancer cell apoptosis requires ER stress activation, but is independent of AMPK.
Fanconi anemia protein FANCD2 is activated by AICAR, a modulator of AMPK and cellular energy metabolism.[Pubmed:28174693]
FEBS Open Bio. 2017 Jan 9;7(2):284-292.
FANCD2 is a pivotal molecule in the pathogenesis of Fanconi anemia (FA), an autosomal recessive human syndrome with diverse clinical phenotypes, including cancer predisposition, short stature, and hematological abnormalities. In our previous study, we detected the functional association of FANC proteins, whose mutations are responsible for the onset of FA, with AMPK in response to DNA interstrand crosslinking lesions. Because AMPK is well known as a critical sensing molecule for cellular energy levels, we checked whether FANCD2 activation occurs after treatments affecting AMPK and/or cellular energy status. Among the treatments tested, AMPK-activating 5-aminoimidazole-4-carboxamide-ribonucleoside (AICAR) induced monoubiquitination and nuclear foci formation of FANCD2, which are biomarkers of FANCD2 activation. FANCD2 activation was abolished by treatments with Compound C, an AMPK inhibitor, or after AMPKalpha1 knockdown, substantiating the involvement of AMPK in AICAR-induced FANCD2 activation. Similarly, FANCA protein, which is a component of the FA core complex monoubiquitinating FANCD2, was required for this event. Furthermore, FANCD2 repression enhanced cell death upon AICAR treatments in transformed fibroblasts and cell cycle arrest in the renal cell carcinoma cell line Caki-1. Overall, this study showed FANCD2 involvement in response to AICAR, a chemical modulating cellular energy metabolism.
AICAR ameliorates high-fat diet-associated pathophysiology in mouse and ex vivo models, independent of adiponectin.[Pubmed:28188334]
Diabetologia. 2017 Apr;60(4):729-739.
AIMS/HYPOTHESIS: In this study, we aimed to evaluate the therapeutic potential of 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), an activator of AMP-activated protein kinase, for ameliorating high-fat diet (HFD)-induced pathophysiology in mice. We also aimed to determine whether the beneficial effects of AICAR were dependent on adiponectin. Furthermore, human adipose tissue was used to examine the effect of AICAR ex vivo. METHODS: Six-week-old male C57BL/6J wild-type and Adipoq (-/-) mice were fed a standard-fat diet (10% fat) or an HFD (60% fat) for 12 weeks and given vehicle or AICAR (500 mug/g) three times/week from weeks 4-12. Diet-induced pathophysiology was examined in mice after 11 weeks by IPGTT and after 12 weeks by flow cytometry and western blotting. Human adipose tissue biopsies from obese (BMI 35-50 kg/m(2)) individuals were incubated with vehicle or AICAR (1 mmol/l) for 6 h at 37 degrees C, after which inflammation was characterised by ELISA (TNF-alpha) and flow cytometry. RESULTS: AICAR attenuated adipose inflammation in mice fed an HFD, promoting an M1-to-M2 macrophage phenotype switch, while reducing infiltration of CD8(+) T cells. AICAR treatment of mice fed an HFD partially restored glucose tolerance and attenuated hepatic steatosis and kidney disease, as evidenced by reduced albuminuria (p < 0.05), urinary H2O2 (p < 0.05) and renal superoxide levels (p < 0.01) in both wild-type and Adipoq (-/-) mice. AICAR-mediated protection occurred independently of adiponectin, as similar protection was observed in wild-type and Adipoq (-/-) mice. In addition, AICAR promoted an M1-to-M2 macrophage phenotype switch and reduced TNF-alpha production in tissue explants from obese human patients. CONCLUSIONS/INTERPRETATION: AICAR may promote metabolic health and protect against obesity-induced systemic diseases in an adiponectin-independent manner. Furthermore, AICAR reduced inflammation in human adipose tissue explants, suggesting by proof-of-principle that the drug may reduce obesity-induced complications in humans. TRIAL REGISTRATION: ClinicalTrials.gov NCT02322073.
AICAR, a small chemical molecule, primes osteogenic differentiation of adult mesenchymal stem cells.[Pubmed:22198598]
Int J Artif Organs. 2011 Dec;34(12):1128-36.
The chemical approach to controlling stem cell fates is emerging as a powerful tool, holding great promise in tissue engineering and regenerative medicine. Various small molecules have been demonstrated capable of modulating stem cell differentiation. In this paper, we studied the effects of 5-aminoimidazole-4-carboxamide-1-ss-riboside (AICAR), an activator of AMP-activated protein kinase (AMPK), on mesenchymal stem cells (MSCs). AICAR at high concentrations (1.0-2.0 mM) significantly inhibited proliferation of both human amnion-derived MSCs (hAMSCs) and rabbit bone marrow-derived MSCs (BM-MSCs). Most importantly, AICAR efficiently promoted the osteogenic differentiation of hAMSCs and BM-MSCs in both growth medium and osteogenic medium. However, Metformin, another AMPK activator, showed no such effects. Meanwhile, AICAR significantly inhibited adipogenic differentiation of hAMSCs and BM-MSCs. Our data suggests that AICAR represents a potent molecule, which can be applied in bone tissue regeneration.
The role of AMPK in psychosine mediated effects on oligodendrocytes and astrocytes: implication for Krabbe disease.[Pubmed:18248608]
J Neurochem. 2008 Jun;105(5):1820-33.
Krabbe disease (KD) is an inherited neurological disorder caused by the deficiency of galactocerebrosidase activity resulting in accumulation of psychosine, which leads to energy depletion, loss of oligodendrocytes, induction of gliosis, and inflammation by astrocytes in CNS. In this study, for the first time, we report the regulation of 'cellular energy switch,' AMP-activated protein kinase (AMPK), by psychosine in oligodendrocytes and astrocytes. Psychosine treatment significantly down-regulated AMPK activity, resulting in increased biosynthesis of lipids including cholesterol and free fatty acid in oligodendrocytes cell line (MO3.13) and primary astrocytes. Pharmacological activator of AMPK, 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) attenuated the psychosine-mediated down-regulation of AMPK and restored altered biosynthesis of lipids. AICAR treatment also down-regulated psychosine induced expression of proinflammatory cytokines and inducible nitric oxide synthase in primary astrocytes. However, AICAR treatment had no effect on psychosine induced-reactive oxygen species generation, arachidonic acid release, and death of oligodendrocytes; suggesting the specific role of AMPK in regulation of psychosine-mediated inflammatory response of astrocytes but not in cell death of oligodendrocytes. This study delineates an explicit role for AMPK in psychosine induced inflammation in astrocytes without directly affecting the cell death of oligodendrocytes. It also suggests that AMPK activating agents act as anti-inflammatory agents and can hold a therapeutic potential in Krabbe disease/twitcher disease, particularly when used in combination with drugs, which protect oligodendrocyte cell loss, such as sPLA2 inhibitor [Giri et al., J. Lipid Res. 47 (2006), 1478].
Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells.[Pubmed:18047638]
BMC Cell Biol. 2007 Nov 29;8:51.
BACKGROUND: Adiponectin is a key mediator of the metabolic syndrome that is caused by visceral fat accumulation. Adiponectin and its receptors are known to be expressed in osteoblasts, but their actions with regard to bone metabolism are still unclear. In this study, we investigated the effects of adiponectin on the proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. RESULTS: Adiponectin receptor type 1 (AdipoR1) mRNA was detected in the cells by RT-PCR. The adenosine monophosphate-activated protein kinase (AMP kinase) was phosphorylated by both adiponectin and a pharmacological AMP kinase activator, 5-amino-imidazole-4-carboxamide-riboside (AICAR), in the cells. AdipoR1 small interfering RNA (siRNA) transfection potently knocked down the receptor mRNA, and the effect of this knockdown persisted for as long as 10 days after the transfection. The transfected cells showed decreased expressions of type I collagen and osteocalcin mRNA, as determined by real-time PCR, and reduced ALP activity and mineralization, as determined by von Kossa and Alizarin red stainings. In contrast, AMP kinase activation by AICAR (0.01-0.5 mM) in wild-type MC3T3-E1 cells augmented their proliferation, differentiation, and mineralization. BrdU assay showed that the addition of adiponectin (0.01-1.0 mug/ml) also promoted their proliferation. Osterix, but not Runx-2, appeared to be involved in these processes because AdipoR1 siRNA transfection and AICAR treatments suppressed and enhanced osterix mRNA expression, respectively. CONCLUSION: Taken together, this study suggests that adiponectin stimulates the proliferation, differentiation, and mineralization of osteoblasts via the AdipoR1 and AMP kinase signaling pathways in autocrine and/or paracrine fashions.
5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase.[Pubmed:14724246]
J Neurosci. 2004 Jan 14;24(2):479-87.
AMP-activated protein kinase (AMPK) is tightly regulated by the cellular AMP:ATP ratio and plays a central role in the regulation of energy homeostasis and metabolic stress. A pharmacological activator of AMPK, 5-amino-4-imidazole carboxamide riboside (AICAR) inhibited lipopolysaccharide (LPS)-induced expression of proinflammatory cytokines (tumor necrosis factor alpha, interleukin-1beta, and interleukin-6) and inducible nitric oxide synthase in primary rat astrocytes, microglia, and peritoneal macrophages. AICAR attenuates the LPS-induced activation of nuclear factor kappaB via downregulation of IkappaB kinase alpha/beta activity. It also inhibits nuclear translocation of CCAAT/enhancer-binding protein (C/EBP) transcription factor by inhibiting the expression of C/EBP-delta in brain glial cells. The dominant negative form of AMPKalpha2 (D157A) and its antisense documents a possible role of AMPK in the regulation of the cellular proinflammatory process. AICAR also inhibited the production of inflammatory mediators in serum and their expression in CNS of rats injected with a sublethal dose of LPS by intraperitoneal injection. These observations in cultured cells as well as in the animal model suggest that AICAR may be of therapeutic value in treating inflammatory diseases.


