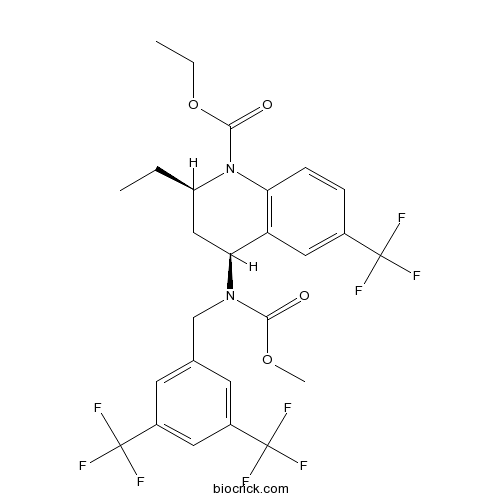
TorcetrapibCETP inhibitor CAS# 262352-17-0 |
- Evacetrapib (LY2484595)
Catalog No.:BCC2329
CAS No.:1186486-62-3
- Dalcetrapib (JTT-705, RO4607381)
Catalog No.:BCC2328
CAS No.:211513-37-0
- Anacetrapib (MK-0859)
Catalog No.:BCC2327
CAS No.:875446-37-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 262352-17-0 | SDF | Download SDF |
| PubChem ID | 159325 | Appearance | Powder |
| Formula | C26H25F9N2O4 | M.Wt | 600.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CP-529414 | ||
| Solubility | DMSO : ≥ 100 mg/mL (166.54 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | ethyl (2R,4S)-4-[[3,5-bis(trifluoromethyl)phenyl]methyl-methoxycarbonylamino]-2-ethyl-6-(trifluoromethyl)-3,4-dihydro-2H-quinoline-1-carboxylate | ||
| SMILES | CCC1CC(C2=C(N1C(=O)OCC)C=CC(=C2)C(F)(F)F)N(CC3=CC(=CC(=C3)C(F)(F)F)C(F)(F)F)C(=O)OC | ||
| Standard InChIKey | CMSGWTNRGKRWGS-NQIIRXRSSA-N | ||
| Standard InChI | InChI=1S/C26H25F9N2O4/c1-4-18-12-21(19-11-15(24(27,28)29)6-7-20(19)37(18)23(39)41-5-2)36(22(38)40-3)13-14-8-16(25(30,31)32)10-17(9-14)26(33,34)35/h6-11,18,21H,4-5,12-13H2,1-3H3/t18-,21+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of cholesteryl ester transfer protein (CETP). Also impairs endothelial function in vivo; induces hypertension and inhibits acetylcholine-induced vasodilation in rabbit central ear artery. |

Torcetrapib Dilution Calculator

Torcetrapib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6654 mL | 8.3268 mL | 16.6536 mL | 33.3072 mL | 41.6341 mL |
| 5 mM | 0.3331 mL | 1.6654 mL | 3.3307 mL | 6.6614 mL | 8.3268 mL |
| 10 mM | 0.1665 mL | 0.8327 mL | 1.6654 mL | 3.3307 mL | 4.1634 mL |
| 50 mM | 0.0333 mL | 0.1665 mL | 0.3331 mL | 0.6661 mL | 0.8327 mL |
| 100 mM | 0.0167 mL | 0.0833 mL | 0.1665 mL | 0.3331 mL | 0.4163 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Torcetrapib is a CETP inhibitor with IC50 of 37 nM, elevates HDL-C and reduces nonHDL-C in plasma. Inhibition of cholesteryl ester transfer protein (CETP) has been shown to have a substantial effect on plasma lipoprotein levels.
In vitro: Torcetrapib dose-dependently increases aldosterone release from H295R cells after either 24 or 48 h of treatment, this effect is mediated by calcium channel as calcium channel blockers completely blocks torcetrapib-induced corticoid release and calcium increase. Torcetrapib (1 μM) significantly increases the expression of steroidogenic gene, CYP11B2 and CYP11B1, in H295R cell lines [1].
In vivo: Researchers tested torcetrapib in rabbits fed an atherogenic diet at a dose sufficient to increase HDL-C by at least 3-fold. CETP activity was inhibited by 70–80% throughout the study. Non-HDL-C increased in both groups, but there was no difference apparent by the study’s end [2].
Clinical trial: Torcetrapib therapy resulted in an increased risk of mortality and morbidity of unknown
mechanism. Although there was evidence of an off-target effect of torcetrapib, adverse effects related to CETP inhibition cannot be ruled out [3].
References:
[1] Hu X, Dietz JD, Xia C, Knight DR, Loging WT, Smith AH, Yuan H, Perry DA, Keiser J. Torcetrapib induces aldosterone and cortisol production by an intracellular calcium-mediated mechanism independently of cholesteryl ester transfer protein inhibition. Endocrinology. 2009;150(5):2211-9.
[2] Morehouse LA, Sugarman ED, Bourassa PA, Sand TM, Zimetti F, Gao F, Rothblat GH, Milici AJ. Inhibition of CETP activity by torcetrapib reduces susceptibility to diet-induced atherosclerosis in New Zealand White rabbits. J Lipid Res. 2007;48(6):1263-72.
[3] Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B; ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109-22.
- Mudanpioside J
Catalog No.:BCC9050
CAS No.:262350-52-7
- Debenzoylgalloylpaeoniflorin
Catalog No.:BCC8927
CAS No.:262350-51-6
- H-D-Aib-OH
Catalog No.:BCC3151
CAS No.:2623-91-8
- 3,6-Dihydroxy-1,7-dimethoxyxanthone
Catalog No.:BCN6394
CAS No.:262292-34-2
- 3alpha-Acetoxy-20-oxo-29-norlupane-23,28-dioic acid
Catalog No.:BCN6507
CAS No.:262272-76-4
- Cl-HOBt
Catalog No.:BCC2829
CAS No.:26198-19-6
- CALP3
Catalog No.:BCC5900
CAS No.:261969-05-5
- CALP2
Catalog No.:BCC5898
CAS No.:261969-04-4
- Isotaxiresinol
Catalog No.:BCN4660
CAS No.:26194-57-0
- SB269970 HCl
Catalog No.:BCC5056
CAS No.:261901-57-9
- Foliamenthoic acid
Catalog No.:BCN5138
CAS No.:26187-80-4
- 6-Prenylsakuranetin
Catalog No.:BCN7883
CAS No.:261776-61-8
- 7,15-Dihydroxypodocarp-8(14)-en-13-one
Catalog No.:BCN1470
CAS No.:262355-96-4
- Cyclofenil
Catalog No.:BCC7839
CAS No.:2624-43-3
- BVD 10
Catalog No.:BCC5882
CAS No.:262418-00-8
- Boc-β-Homo-Pro-OH
Catalog No.:BCC2628
CAS No.:26250-84-0
- Peritassine A
Catalog No.:BCC9117
CAS No.:262601-67-2
- AICAR
Catalog No.:BCC3606
CAS No.:2627-69-2
- 2,3',4,6-Tetrahydroxybenzophenone
Catalog No.:BCN5139
CAS No.:26271-33-0
- Ficaprenol 11
Catalog No.:BCN5140
CAS No.:26296-50-4
- Physcion-8-O-beta-D-monoglucoside
Catalog No.:BCN8511
CAS No.:26296-54-8
- Pepstatin A
Catalog No.:BCC1218
CAS No.:26305-03-3
- 22-Dehydroclerosterol
Catalog No.:BCN5141
CAS No.:26315-07-1
- Dehydroperilloxin
Catalog No.:BCN7506
CAS No.:263241-09-4
Modulating Drug Release and Enhancing the Oral Bioavailability of Torcetrapib with Solid Lipid Dispersion Formulations.[Pubmed:25690735]
AAPS PharmSciTech. 2015 Oct;16(5):1091-100.
The development of drug dispersions using solid lipids is a novel formulation strategy that can help address the challenges of poor drug solubility and systemic exposure after oral administration. The highly lipophilic and poorly water-soluble drug Torcetrapib could be effectively formulated into solid lipid microparticles (SLMs) using an anti-solvent precipitation strategy. Acoustic milling was subsequently used to obtain solid lipid nanoparticles (SLNs). Torcetrapib was successfully incorporated into the lipid matrix in an amorphous state. Spherical SLMs with mean particle size of approximately 15-18 mum were produced with high drug encapsulation efficiency (>96%) while SLNs were produced with a mean particle size of 155 nm and excellent colloidal stability. The in vitro drug release and the in vivo absorption of the solid lipid micro- and nanoparticles after oral dosing in rats were evaluated against conventional crystalline drug powders as well as a spray dried amorphous polymer dispersion formulation. Interestingly, the in vitro drug release rate from the lipid particles could be tuned for immediate or extended release by controlling either the particle size or the precipitation temperature used when forming the drug-lipid particles. This change in the rate of drug release was manifested in vivo with changes in Tmax as well. In addition, in vivo pharmacokinetic studies revealed a significant increase ( approximately 6 to 11-fold) in oral bioavailability in rats dosed with the SLMs and SLNs compared to conventional drug powders. Importantly, this formulation approach can be performed rapidly on a small scale, making it ideal as a formulation technology for use early in the drug discovery timeframe.
Raising HDL with CETP inhibitor torcetrapib improves glucose homeostasis in dyslipidemic and insulin resistant hamsters.[Pubmed:24530763]
Atherosclerosis. 2014 Apr;233(2):359-62.
We investigated whether raising HDL-cholesterol levels with cholesteryl ester transfer protein (CETP) inhibition improves glucose homeostasis in dyslipidemic and insulin resistant hamsters. Compared with vehicle, Torcetrapib 30 mg/kg/day (TOR) administered for 10 days significantly increased by approximately 40% both HDL-cholesterol levels and 3H-tracer appearance in HDL after 3H-cholesterol labeled macrophages i.p. injection. TOR significantly reduced fasting plasma triglycerides, glycerol and free fatty acids levels by 65%, 31% and 23%, respectively. TOR also reduced blood glucose levels and plasma insulin by 20% and 49% respectively, which led to a 60% reduction in HOMA-IR index (all p<0.01). After 3H-2-deoxyglucose and insulin injection, TOR significantly increased glucose uptake in oxidative soleus muscle, liver and heart by 26, 33 and 70%, respectively. Raising HDL levels with the CETP inhibitor Torcetrapib improves glucose homeostasis in dyslipidemic and insulin resistant hamsters. Whether similar effect would be observed with other CETP inhibitors should be investigated.
Identification of a novel, non-tetrahydroquinoline variant of the cholesteryl ester transfer protein (CETP) inhibitor torcetrapib, with improved aqueous solubility.[Pubmed:24380613]
Xenobiotica. 2014 Jul;44(7):591-605.
1. Elaborate studies of cholesteryl ester transfer protein (CETP) polymorphisms and genetic deficiency in humans suggest direct links between CETP, high-density lipoprotein cholesterol (HDL-c) levels and coronary heart diseases. The hypothesis that CETP inhibition by small molecule inhibitors raises HDL-c has been validated clinically with structurally-diverse CETP inhibitors such as Torcetrapib, anacetrapib, dalcetrapib and evacetrapib. 2. Despite promising phase 2 results with respect to HDL-c elevation, Torcetrapib was discontinued in phase 3 trials due to increased mortality rates in the cardiovascular outcomes study. Emerging evidence for the adverse effects hints at off-target chemotype-specific cardiovascular toxicity, possibly related to the pressor effects of Torcetrapib, since structurally diverse CETP inhibitors such as anacetrapib, evacetrapib and dalcetrapib are not associated with blood pressure increases in humans. Nonclinical follow-up studies showed that Torcetrapib induces aldosterone biosynthesis and secretion in vivo and in vitro, an effect which is not observed with other CETP inhibitors in clinical development. 3. As part of ongoing efforts to identify novel CETP inhibitors devoid of pressor effects, strategies were implemented towards the design of compounds, which lack the 1,2,3,4-tetrahydroquinoline (THQ) scaffold present in Torcetrapib. In this article, we disclose results of structure-activity relationship studies for a series of novel non-THQ CETP inhibitors, which resulted in the identification of a novel isonipecotic acid derivative 10 (also referred to as PF-04445597) with vastly improved oral pharmacokinetic properties mainly as a result of improved aqueous solubility. This feature is attractive in that, it bypasses significant investments needed to develop compatible solubilizing formulation(s) for oral drug delivery of highly lipophilic and poorly soluble compounds; attributes, which are usually associated with small molecule CETP inhibitors. PF-04445597 was also devoid of aldosterone secretion in human H295R adrenal carcinoma cells.
In vitro-in vivo evaluation of lipid based formulations of the CETP inhibitors CP-529,414 (torcetrapib) and CP-532,623.[Pubmed:25152213]
Eur J Pharm Biopharm. 2014 Nov;88(3):973-85.
The present study investigated the use of lipid based drug delivery systems to enhance the oral bioavailability of the CETP inhibitors CP-532,623 and Torcetrapib. A series of self-emulsifying lipid based drug delivery systems (SEDDS) were assembled and examined using an in vitro lipid digestion model to evaluate patterns of drug precipitation under simulated intestinal conditions. Drug exposure after oral administration of the same formulations was subsequently assessed in beagle dogs. CP-532,623 was maintained in a solubilised state during dispersion of most formulations in simulated intestinal fluid, however, solubilisation capacity was reduced to various degrees upon in vitro digestion. Administration of SEDDS formulations to beagle dogs resulted in moderate differences in plasma AUC when compared to the differences in solubilisation observed in vitro. Similar trends were observed for Torcetrapib. In all cases, however, in vivo exposure of CP-532,623 was greatly enhanced by administration in lipid based drug delivery systems when compared to a powder formulation. Some correlation between in vitro solubilisation and in vivo drug exposure (AUC) was evident; however, this was not linear. The data suggest that for highly lipophilic drugs such as CP-532,623 in vitro digestion data may be a conservative in vitro indicator of utility and that good exposure may be evident even for formulations that result in significant drug precipitation during in vitro digestion.
Torcetrapib produces endothelial dysfunction independent of cholesteryl ester transfer protein inhibition.[Pubmed:20051879]
J Cardiovasc Pharmacol. 2010 May;55(5):459-68.
OBJECTIVE: Torcetrapib, a prototype cholesteryl ester transfer protein (CETP) inhibitor with potential for decreasing atherosclerotic disease, increased cardiovascular events in clinical trials. The identified hypertensive and aldosterone-elevating actions of Torcetrapib may not fully account for this elevated cardiovascular risk. Therefore, we evaluated the effects of Torcetrapib on endothelial mediated vasodilation in vivo. METHODS AND RESULTS: In vivo endothelial mediated vasodilation was assessed using ultrasound imaging of acetylcholine-induced changes in rabbit central ear artery diameter. Torcetrapib, in addition to producing hypertension and baseline vasoconstriction, markedly inhibited acetylcholine-induced vasodilation. A structurally distinct CETP inhibitor, JNJ-28545595, did not affect endothelial function despite producing similar degrees of CETP inhibition and high-density lipoprotein elevation. Nitroprusside normalized Torcetrapib's basal vasoconstriction and elicited dose-dependent vasodilation of norepinephrine preconstricted arteries in Torcetrapib-treated animals, indicating Torcetrapib did not impair smooth muscle function. CONCLUSIONS: Torcetrapib significantly impairs endothelial function in vivo, independent of CETP inhibition and high-density lipoprotein elevation. Given the well-documented association of endothelial dysfunction with cardiovascular disease and risk, this activity of Torcetrapib may have contributed to increased cardiovascular risk in clinical trials.
Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone.[Pubmed:18536749]
Br J Pharmacol. 2008 Aug;154(7):1465-73.
BACKGROUND AND PURPOSE: Inhibition of cholesteryl ester transfer protein (CETP) with Torcetrapib in humans increases plasma high density lipoprotein (HDL) cholesterol levels but is associated with increased blood pressure. In a phase 3 clinical study, evaluating the effects of Torcetrapib in atherosclerosis, there was an excess of deaths and adverse cardiovascular events in patients taking Torcetrapib. The studies reported herein sought to evaluate off-target effects of Torcetrapib. EXPERIMENTAL APPROACH: Cardiovascular effects of the CETP inhibitors Torcetrapib and anacetrapib were evaluated in animal models. KEY RESULTS: Torcetrapib evoked an acute increase in blood pressure in all species evaluated whereas no increase was observed with anacetrapib. The pressor effect of Torcetrapib was not diminished in the presence of adrenoceptor, angiotensin II or endothelin receptor antagonists. Torcetrapib did not have a contractile effect on vascular smooth muscle suggesting its effects in vivo are via the release of a secondary mediator. Treatment with Torcetrapib was associated with an increase in plasma levels of aldosterone and corticosterone and, in vitro, was shown to release aldosterone from adrenocortical cells. Increased adrenal steroid levels were not observed with anacetrapib. Inhibition of adrenal steroid synthesis did not inhibit the pressor response to Torcetrapib whereas adrenalectomy prevented the ability of Torcetrapib to increase blood pressure in rats. CONCLUSIONS AND IMPLICATIONS: Torcetrapib evoked an acute increase in blood pressure and an acute increase in plasma adrenal steroids. The acute pressor response to Torcetrapib was not mediated by adrenal steroids but was dependent on intact adrenal glands.


