Evacetrapib (LY2484595)CETP inhibitor,potent and selective CAS# 1186486-62-3 |
- Ritonavir
Catalog No.:BCC3620
CAS No.:155213-67-5
- Amprenavir (agenerase)
Catalog No.:BCC3619
CAS No.:161814-49-9
- Lopinavir
Catalog No.:BCC3621
CAS No.:192725-17-0
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- Darunavir
Catalog No.:BCC3623
CAS No.:206361-99-1
- Maraviroc
Catalog No.:BCC3675
CAS No.:376348-65-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1186486-62-3 | SDF | Download SDF |
| PubChem ID | 49836058 | Appearance | Powder |
| Formula | C31H36F6N6O2 | M.Wt | 638.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | LY2484595 | ||
| Solubility | Soluble in DMSO > 10 mM | ||
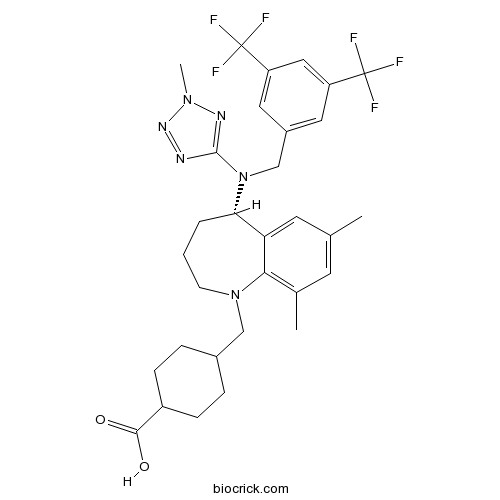
| Chemical Name | 4-[[(5S)-5-[[3,5-bis(trifluoromethyl)phenyl]methyl-(2-methyltetrazol-5-yl)amino]-7,9-dimethyl-2,3,4,5-tetrahydro-1-benzazepin-1-yl]methyl]cyclohexane-1-carboxylic acid | ||
| SMILES | CC1=CC(=C2C(=C1)C(CCCN2CC3CCC(CC3)C(=O)O)N(CC4=CC(=CC(=C4)C(F)(F)F)C(F)(F)F)C5=NN(N=N5)C)C | ||
| Standard InChIKey | IHIUGIVXARLYHP-UXNJHFGPSA-N | ||
| Standard InChI | InChI=1S/C31H36F6N6O2/c1-18-11-19(2)27-25(12-18)26(5-4-10-42(27)16-20-6-8-22(9-7-20)28(44)45)43(29-38-40-41(3)39-29)17-21-13-23(30(32,33)34)15-24(14-21)31(35,36)37/h11-15,20,22,26H,4-10,16-17H2,1-3H3,(H,44,45)/t20?,22?,26-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Evacetrapib is a potent and selective inhibitor of cholesterylester transfer protein (CETP) with IC50 value of 5.5 nM. | |||||
| Targets | CETP | |||||
| IC50 | 5.5 nM | |||||

Evacetrapib (LY2484595) Dilution Calculator

Evacetrapib (LY2484595) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5658 mL | 7.829 mL | 15.658 mL | 31.3161 mL | 39.1451 mL |
| 5 mM | 0.3132 mL | 1.5658 mL | 3.1316 mL | 6.2632 mL | 7.829 mL |
| 10 mM | 0.1566 mL | 0.7829 mL | 1.5658 mL | 3.1316 mL | 3.9145 mL |
| 50 mM | 0.0313 mL | 0.1566 mL | 0.3132 mL | 0.6263 mL | 0.7829 mL |
| 100 mM | 0.0157 mL | 0.0783 mL | 0.1566 mL | 0.3132 mL | 0.3915 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Evacetrapib is a potent and selective inhibitor of cholesteryl ester transfer protein (CETP) with IC50 value of 5.5nM [1].
As a benzazepine-based inhibitor of CETP, evacetrapib is developed to increase HDL cholesterol for the treatment of coronary artery disease. Evacetrapib is efficacious both in vitro and in vivo. The IC50 values of evacetrapib against CETP are 5.5nM and 26nM, respectively in the buffer assay using human recombinant CETP and in the plasma assay using human plasma CETP. In the animal model of human CETP/ApoAI double transgenic mouse line, oral administration of evacetrapib at 30mg/kg significantly inhibits the CETP activity as well as increases the level of HDL. Besides that, the ED50 value of evacetrapib is less than 5mg/kg. Compared to the previous inhibitors of CETP, evacetrapib has less side effects. It is found no to increase blood pressure Zucker diabetic fatty rats and not to induce aldosterone or cortisol synthesis in H295R cells [1].
References:
[1] Cao G, Beyer TP, Zhang Y, Schmidt RJ, Chen YQ, Cockerham SL, Zimmerman KM, Karathanasis SK, Cannady EA, Fields T, Mantlo NB. Evacetrapib is a novel, potent, and selective inhibitor of cholesteryl ester transfer protein that elevates HDL cholesterol without inducing aldosterone or increasing blood pressure. J Lipid Res. 2011 Dec;52(12):2169-76.
- TMN 355
Catalog No.:BCC6121
CAS No.:1186372-20-2
- Epivogeloside
Catalog No.:BCN6060
CAS No.:118627-52-4
- 4SC-202
Catalog No.:BCC5359
CAS No.:1186222-89-8
- ALW-II-41-27
Catalog No.:BCC1350
CAS No.:1186206-79-0
- NPE-caged-proton
Catalog No.:BCC7698
CAS No.:1186195-63-0
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- Tocrifluor T1117
Catalog No.:BCC7401
CAS No.:1186195-59-4
- BU 226 hydrochloride
Catalog No.:BCC6936
CAS No.:1186195-56-1
- Vitexdoin A
Catalog No.:BCN4089
CAS No.:1186021-77-1
- VD3-D6
Catalog No.:BCC4076
CAS No.:118584-54-6
- NVP-BVU972
Catalog No.:BCC3828
CAS No.:1185763-69-2
- Zileuton sodium
Catalog No.:BCC4216
CAS No.:118569-21-4
- 11-Hydroxycodaphniphylline
Catalog No.:BCN6061
CAS No.:1186496-68-3
- H-Cys(Me)-OH
Catalog No.:BCC2908
CAS No.:1187-84-4
- Resminostat hydrochloride
Catalog No.:BCC1888
CAS No.:1187075-34-8
- Cuniloside B
Catalog No.:BCN6062
CAS No.:1187303-40-7
- Trametinib DMSO solvate
Catalog No.:BCC2013
CAS No.:1187431-43-1
- PF 184
Catalog No.:BCC6130
CAS No.:1187460-81-6
- Baricitinib (LY3009104, INCB028050)
Catalog No.:BCC2195
CAS No.:1187594-09-7
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- Carabrolactone A
Catalog No.:BCN6063
CAS No.:1187925-30-9
- Carabrolactone B
Catalog No.:BCN6064
CAS No.:1187925-31-0
- Diosbulbin I
Catalog No.:BCN6065
CAS No.:1187951-05-8
- Diosbulbin J
Catalog No.:BCN6066
CAS No.:1187951-06-9
Efficacy and Safety of the Cholesteryl Ester Transfer Protein Inhibitor Evacetrapib in Combination With Atorvastatin in Japanese Patients With Primary Hypercholesterolemia.[Pubmed:28768921]
Circ J. 2017 Dec 25;82(1):183-191.
BACKGROUND: Inhibition of cholesteryl ester transfer protein by evacetrapib when added to atorvastatin may provide an additional treatment option for patients who do not reach their low-density lipoprotein cholesterol (LDL-C) goal.Methods and Results:This multicenter, randomized, 12-week, double-blind, parallel-group, placebo-controlled, outpatient, phase 3 study evaluated the efficacy of evacetrapib with atorvastatin in reducing LDL-C in 149 Japanese patients (evacetrapib/atorvastatin, n=53; ezetimibe/atorvastatin, n=50; placebo/atorvastatin, n=46) with primary hypercholesterolemia. The primary efficacy measure was percent change from baseline to week 12 in LDL-C (beta quantification). Treatment with evacetrapib 130 mg daily for 12 weeks resulted in a statistically significant treatment difference of -25.70% compared with placebo in percentage decrease in LDL-C (95% CI: -34.73 to -16.68; P<0.001). Treatment with evacetrapib 130 mg also resulted in a statistically significant difference of 126.39% in the change in high-density lipoprotein cholesterol (HDL-C) compared with placebo (95% CI: 113.54-139.24; P<0.001). No deaths or serious adverse events were reported. Four patients (3 in the evacetrapib group and 1 in the ezetimibe group) discontinued due to adverse events. CONCLUSIONS: Evacetrapib daily in combination with atorvastatin was superior to placebo in lowering LDL-C after 12 weeks, and resulted in a statistically significant increase of HDL-C compared with placebo. Also, no new safety risks were identified.
No cardiovascular benefit with evacetrapib - is this the end of the road for the 'cetrapibs'?[Pubmed:28799819]
Expert Opin Pharmacother. 2017 Oct;18(14):1439-1442.
INTRODUCTION: Increasing high-density lipoprotein(HDL) cholesterol levels predict improved cardiovascular outcomes. However, inhibiting cholesteryl ester transfer protein (CETP) to increase HDL cholesterol, with the 'cetrapibs' (torcetrapib and dalcetrapib), did not improve cardiovascular clinical outcomes. Despite these findings, the clinical outcomes trial with evacetrapib continued. Areas covered: Treatment with evacetrapib increased the levels of HDL by ~130%, and decreased low-density lipoprotein (LDL) cholesterol by ~37%. However, The Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition with Evacetrapib in Patients at a High Risk for Vascular Outcomes (ACCELERATE) trial did not show reduced cardiovascular outcomes with this cetrapib. Evacetrapib may have failed because increasing HDL cholesterol may not be beneficial in the presence of coronary artery disease and/or it is possible that evacetrapib has toxic effects that counter any beneficial effects. Expert opinion: In addition to our understanding of the relationships between CETP, HDL cholesterol and cardiovascular disease being incomplete, recent meta-analysis evidence suggests that increasing HDL cholesterol does not improve cardiovascular outcomes in subjects taking statins, and this may explain the failure of evacetrapib. Also, the preclinical characteristics of the cetrapibs, especially off-target mechanisms, were not explored prior to clinical trial, and may have contributed to the failure of cetrapibs, including evacetrapib.
Binding profiles of cholesterol ester transfer protein with current inhibitors: a look at mechanism and drawback.[Pubmed:28777919]
J Biomol Struct Dyn. 2018 Aug;36(10):2567-2580.
Although the pharmacological inhibition of cholesterol ester transport protein (CETP) has been proposed as a method of preventing and treating cardiovascular disease (CVD), the adverse effects of current inhibitors have cast doubt on the interaction mechanisms of inhibitors and CETP. In response, a molecular dynamics simulation was used to investigate their interaction and shed light on the lipid exchange mechanism of CETP. Results showed that torcetrapib, anacetrapib, and evacetrapib can induce the incremental rigidity of CETP, yet decrease the stability of Helix X and the hydrophobic tunnel of CETP, with passable binding abilities (DeltaGbind, -61.08, -64.23, and -61.57 kcal mol(-1)). During their binding processes, Van der Waals components (DeltaEvdw + DeltaGSA) play a dominant role, and the inhibitory effects closely correlated with residues Cys13, Val198, Gln199, Ser230, His232, and Phe263, which could reduce the flexibility of N- and C- termini and Helix X, as well as the stability of hydrophobic tunnel, into which the three inhibitors could enter and promote the formation of intramolecular H-bonds such as Thr138-Asn192 and Arg37-Glu186. Additionally, the three inhibitors could restrain the formation of an opening at the CETP N-terminal, which given the other findings suggests the tunneling mechanism of CETP transfer. The paper closes with an explanation of conceivable causes of the insufficient efficacy of the inhibitors, and puts forward the rationality in targeting the CETP distal end for CVD therapies.


