H-Cys(Me)-OHCAS# 1187-84-4 |
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1187-84-4 | SDF | Download SDF |
| PubChem ID | 24417 | Appearance | Powder |
| Formula | C4H9NO2S | M.Wt | 135.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | L-S-Methylcysteine | ||
| Solubility | H2O : 30 mg/mL (221.93 mM; Need ultrasonic and warming) | ||
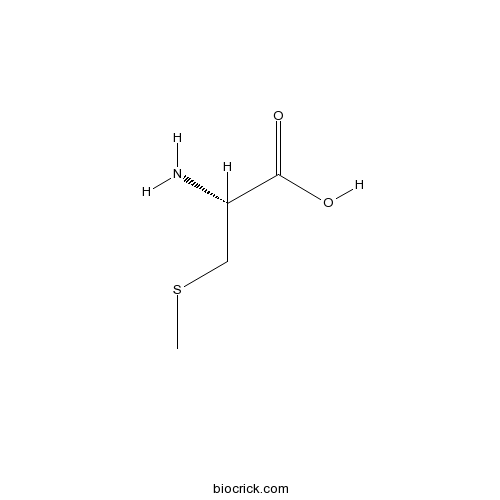
| Chemical Name | (2R)-2-amino-3-methylsulfanylpropanoic acid | ||
| SMILES | CSCC(C(=O)O)N | ||
| Standard InChIKey | IDIDJDIHTAOVLG-VKHMYHEASA-N | ||
| Standard InChI | InChI=1S/C4H9NO2S/c1-8-2-3(5)4(6)7/h3H,2,5H2,1H3,(H,6,7)/t3-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | S-Methyl-L-cysteine is a natural product that acts as a substrate in the catalytic antioxidant system mediated by methionine sulfoxide reductase A (MSRA), with antioxidative, neuroprotective, and anti-obesity activities.In Vivo:S-Methyl-L-cysteine (100 mg/kg) results in significant attenuation of plasma glucose, insulin, tumor necrosis factor-alpha, insulin resistance and improved antioxidant enzyme activities[1]. References: | |||||

H-Cys(Me)-OH Dilution Calculator

H-Cys(Me)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.3964 mL | 36.9822 mL | 73.9645 mL | 147.929 mL | 184.9112 mL |
| 5 mM | 1.4793 mL | 7.3964 mL | 14.7929 mL | 29.5858 mL | 36.9822 mL |
| 10 mM | 0.7396 mL | 3.6982 mL | 7.3964 mL | 14.7929 mL | 18.4911 mL |
| 50 mM | 0.1479 mL | 0.7396 mL | 1.4793 mL | 2.9586 mL | 3.6982 mL |
| 100 mM | 0.074 mL | 0.3698 mL | 0.7396 mL | 1.4793 mL | 1.8491 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H-Cys(Me)-OH
- 11-Hydroxycodaphniphylline
Catalog No.:BCN6061
CAS No.:1186496-68-3
- Evacetrapib (LY2484595)
Catalog No.:BCC2329
CAS No.:1186486-62-3
- TMN 355
Catalog No.:BCC6121
CAS No.:1186372-20-2
- Epivogeloside
Catalog No.:BCN6060
CAS No.:118627-52-4
- 4SC-202
Catalog No.:BCC5359
CAS No.:1186222-89-8
- ALW-II-41-27
Catalog No.:BCC1350
CAS No.:1186206-79-0
- NPE-caged-proton
Catalog No.:BCC7698
CAS No.:1186195-63-0
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- Tocrifluor T1117
Catalog No.:BCC7401
CAS No.:1186195-59-4
- BU 226 hydrochloride
Catalog No.:BCC6936
CAS No.:1186195-56-1
- Vitexdoin A
Catalog No.:BCN4089
CAS No.:1186021-77-1
- VD3-D6
Catalog No.:BCC4076
CAS No.:118584-54-6
- Resminostat hydrochloride
Catalog No.:BCC1888
CAS No.:1187075-34-8
- Cuniloside B
Catalog No.:BCN6062
CAS No.:1187303-40-7
- Trametinib DMSO solvate
Catalog No.:BCC2013
CAS No.:1187431-43-1
- PF 184
Catalog No.:BCC6130
CAS No.:1187460-81-6
- Baricitinib (LY3009104, INCB028050)
Catalog No.:BCC2195
CAS No.:1187594-09-7
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- Carabrolactone A
Catalog No.:BCN6063
CAS No.:1187925-30-9
- Carabrolactone B
Catalog No.:BCN6064
CAS No.:1187925-31-0
- Diosbulbin I
Catalog No.:BCN6065
CAS No.:1187951-05-8
- Diosbulbin J
Catalog No.:BCN6066
CAS No.:1187951-06-9
- Ac-Leu-OH
Catalog No.:BCC2967
CAS No.:1188-21-2
- ent-14,16-Epoxy-8-pimarene-3,15-diol
Catalog No.:BCN6067
CAS No.:1188281-98-2
Cytotoxic T lymphocyte epitope analogues containing cis- or trans-4-aminocyclohexanecarboxylic acid residues.[Pubmed:12110330]
Bioorg Med Chem. 2002 Sep;10(9):3061-6.
In order to improve the immunotherapeutical potential of H-Cys-Leu-Gly-Gly-Leu-Leu-Thr-Met-Val-OH (CLG) peptide, an Epstein-Barr virus (EBV) subdominant epitope derived from the membrane protein LMP2, we have synthesized and tested CLG analogues containing cis- and/or trans-4-aminocyclohexanecarboxylic acid (ACCA) replacing Gly-Gly and/or Thr-Met dipeptide units. All pseudopeptides were tested for metabolic stability and for their capacity to bind HLA-A2 molecules and to sensitize target cells to lysis. All new compounds exhibited higher enzymatic resistance compared to the original CLG and some trans-ACCA-derivatives were able to associate HLA-A2 and to efficiently stimulate CTL responses directed against the CLG natural epitope.
Conformational analysis of a potent SSTR3-selective somatostatin analogue by NMR in water solution.[Pubmed:16365912]
J Pept Sci. 2006 Feb;12(2):82-91.
The three-dimensional structure of a potent SSTR3-selective analogue of somatostatin, cyclo(3-14)H-Cys(3)-Phe(6)-Tyr(7)-D-Agl(8)(N(beta) Me, 2-naphthoyl)-Lys(9)-Thr(10)-Phe(11)-Cys(14)-OH (des-AA(1, 2, 4, 5, 12, 13)[Tyr(7), D-Agl(8)(N(beta) Me, 2-naphthoyl)]-SRIF) (peptide 1) has been determined by (1)H NMR in water and molecular dynamics (MD) simulations. The peptide exists in two conformational isomers differing mainly by the cis/trans isomerization of the side chain in residue 8. The structure of 1 is compared with the consensus structural motifs of other somatostatin analogues that bind predominantly to SSTR1, SSTR2/SSTR5 and SSTR4 receptors, and to the 3D structure of a non-selective SRIF analogue, cyclo(3-14)H-Cys(3)-Phe(6)-Tyr(7)-D-2Nal(8)-Lys(9)-Thr(10)-Phe(11)-Cys(14)-OH (des-AA(1, 2, 4, 5, 12, 13)[Tyr(7), D-2Nal(8)]-SRIF) (peptide 2). The structural determinant factors that could explain selectivity of peptide 1 for SSTR3 receptors are discussed.
Synthetic beta-endorphin-like peptide immunorphin binds to non-opioid receptors for beta-endorphin on T lymphocytes.[Pubmed:11786184]
Peptides. 2001 Dec;22(12):2009-13.
The synthetic decapeptide H-SLTCLVKGFY-OH (termed immunorphin) corresponding to the sequence 364-373 of the CH3 domain of human immunoglobulin G heavy chain was found to compete with [125I]beta-endorphin for high-affinity receptors on T lymphocytes from the blood of healthy donors (K(i) = 0.6 nM). Besides immunorphin, its synthetic fragments H-Val-Lys-Gly-Phe-Tyr-OH (K(i) = 15 nM), H-Leu-Val-Lys-Gly-Phe-Tyr-OH (K(i) = 8.0 nM), H-Cys-Leu-Val-Lys-Gly-Phe-Tyr-OH (K(i) = 3.4 nM), H-Thr-Cys-Leu-Val-Lys-Gly-Phe-Tyr-OH (K(i) = 2.2 nM), H-Leu-Thr-Cys-Leu-Val-Lys-Gly-Phe-Tyr-OH (K(i) = 1.0 nM) possessed the ability to inhibit specific binding of [125I]beta-endorphin to T lymphocytes. Tests of the specificity of the receptors revealed that they are not sensitive to naloxone and Met-enkephalin, i.e. they are not opioid receptors. K(d) values characterizing the specific binding of 125I- labeled immunorphin and its fragment H-Val-Lys-Gly-Phe-Tyr-OH to the receptors have been determined to be 7.4 nM and 36.3 nM, respectively.
N-imidazolebenzyl-histidine substitution in somatostatin and in its octapeptide analogue modulates receptor selectivity and function.[Pubmed:21806016]
J Med Chem. 2011 Sep 8;54(17):5981-7.
Despite 3 decades of focused chemical, biological, structural, and clinical developments, unusual properties of somatostatin (SRIF, 1) analogues are still being uncovered. Here we report the unexpected functional properties of 1 and the octapeptide cyclo(3-14)H-Cys-Phe-Phe-Trp(8)-Lys-Thr-Phe-Cys-OH (somatostatin numbering; OLT-8, 9) substituted by imBzl-l- or -d-His at position 8. These analogues were tested for their binding affinity to the five human somatostatin receptors (sst(1-5)), as well as for their functional properties (or functionalities) in an sst(3) internalization assay and in an sst(3) luciferase reporter gene assay. While substitution of Trp(8) in somatostatin by imBzl-l- or -d-His(8) results in sst(3) selectivity, substitution of Trp(8) in the octapeptide 9 by imBzl-l- or -d-His(8) results in loss of binding affinity for sst(1,2,4,5) and a radical functional switch from agonist to antagonist.
Synthesis, biological activity, and solution structures of a cyclic dodecapeptide from the EGF-2 domain of blood coagulation factor VII.[Pubmed:11437950]
J Pept Res. 2001 Jun;57(6):462-72.
The cyclic dodecapeptide, disulfide-cyclo-[H-Cys-Val-Asn-Glu-Asn-Gly-Gly-Cys(Acm)-Glu-Gln-Tyr-Cys-OH], which corresponds to the 91-102 sequence of the second epidermal growth factor domain of human blood coagulation factor VII, was synthesized using solid-phase procedures. It was shown to be an inhibitor at the key step in the induction of coagulation by the extrinsic pathway, i.e. the factor VII/tissue factor-catalyzed activation of coagulation factor X. The solution structure of this peptide was investigated by NMR spectroscopy and was computer-modeled via molecular mechanics. Structures were calculated based on 112 distance and nine dihedral angle constraints. The resulting backbone structures were classified into two structural subsets: one which exhibited a twisted '8'-shaped folding and another describing an open, circular 'O' outline. The local backbone structures of segments Asn3-Glu4-Asn5, Gly7-Cys8 and Gln10-Tyr11 were well preserved among the two subsets. Apart from the unrestrained N- and C-termini, Gly6 and Glu9 sites exhibited marked local disorder between the two subsets, suggesting localized flexible hinges likely to govern tertiary structure interconversion between the two subsets. Two transient hydrogen bonds were identified from pH chemical shift titrations by matching the pKa values of NH and carboxylate groups, which supported the occurrence of the '8' structure, and agreed with temperature coefficients of peptidyl NH resonances. Structure-function relationships of the peptide were discussed in terms of the likely physiological function of the disulfide-bonded loop in factor VII which the peptide represents.


