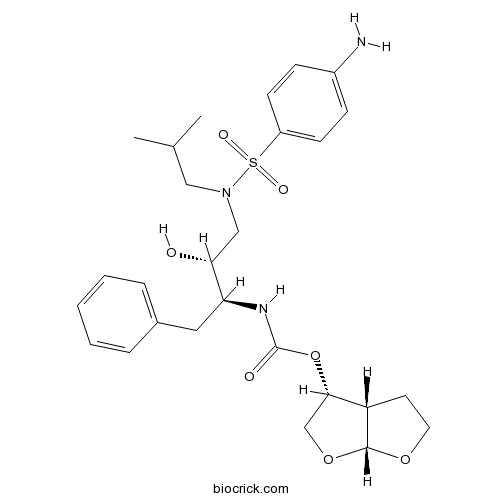
DarunavirHIV-1 protease inhibitor CAS# 206361-99-1 |
- Saquinavir
Catalog No.:BCC1921
CAS No.:127779-20-8
- Saquinavir mesylate
Catalog No.:BCC1922
CAS No.:149845-06-7
- Ritonavir
Catalog No.:BCC3620
CAS No.:155213-67-5
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Tipranavir
Catalog No.:BCC2002
CAS No.:174484-41-4
- Lopinavir
Catalog No.:BCC3621
CAS No.:192725-17-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 206361-99-1 | SDF | Download SDF |
| PubChem ID | 213039 | Appearance | Powder |
| Formula | C27H37N3O7S | M.Wt | 547.66 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | TMC114 | ||
| Solubility | DMSO : ≥ 100 mg/mL (182.60 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [(3aS,4R,6aR)-2,3,3a,4,5,6a-hexahydrofuro[2,3-b]furan-4-yl] N-[(2S,3R)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl]carbamate | ||
| SMILES | CC(C)CN(CC(C(CC1=CC=CC=C1)NC(=O)OC2COC3C2CCO3)O)S(=O)(=O)C4=CC=C(C=C4)N | ||
| Standard InChIKey | CJBJHOAVZSMMDJ-HEXNFIEUSA-N | ||
| Standard InChI | InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Darunavir is an inhibitor of HIV protease. | |||||
| Targets | HIV Protease | |||||

Darunavir Dilution Calculator

Darunavir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.826 mL | 9.1298 mL | 18.2595 mL | 36.519 mL | 45.6488 mL |
| 5 mM | 0.3652 mL | 1.826 mL | 3.6519 mL | 7.3038 mL | 9.1298 mL |
| 10 mM | 0.1826 mL | 0.913 mL | 1.826 mL | 3.6519 mL | 4.5649 mL |
| 50 mM | 0.0365 mL | 0.1826 mL | 0.3652 mL | 0.7304 mL | 0.913 mL |
| 100 mM | 0.0183 mL | 0.0913 mL | 0.1826 mL | 0.3652 mL | 0.4565 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Darunavir is an orally-bioavailable non-peptidic inhibitor of human immunodeficiency virus type 1 (HIV-1) protease that selectively inhibits HIV-1 protease enzyme induced cleavage of gag and gag-pol poly-proteins preventing the maturation of virions and also inhibits the dimerization of HIV-1 protease suppressing proteolytic activity and subsequent replication of HIV-1. Study results have shown that darunavir exhibits potent inhibition against HIV-1 with a value of 50% inhibition concentration IC50 of 0.003 μmol/L in HIV-1 infected MT-2 cells. Darunavir binds to HIV-1 protease with considerably high affinity and fits closely with the substrate envelop leading to its potent ability to inhibit multidrug-resistant HIV strains.
Reference
McKeage K, Perry CM, Keam SJ. Darunavir: a review of its use in the management of HIV infection in adults. Drugs. 2009;69(4):477-503. doi: 10.2165/00003495-200969040-00007.
- L-R4W2
Catalog No.:BCC5779
CAS No.:206350-79-0
- Monomelittoside
Catalog No.:BCN8509
CAS No.:20633-72-1
- Calycosin-7-O-beta-D-glucoside
Catalog No.:BCN5931
CAS No.:20633-67-4
- Encecalin
Catalog No.:BCN4898
CAS No.:20628-09-5
- CB30865
Catalog No.:BCC1457
CAS No.:206275-15-2
- Tenofovir hydrate
Catalog No.:BCC4261
CAS No.:206184-49-8
- Ergosterol peroxide
Catalog No.:BCN4897
CAS No.:2061-64-5
- Tetrahydromagnolol
Catalog No.:BCN8255
CAS No.:20601-85-8
- H-D-Asn-OH.H2O
Catalog No.:BCC2879
CAS No.:2058-58-4
- Oxytetracycline hydrochloride
Catalog No.:BCC9110
CAS No.:2058-46-0
- Calycosin
Catalog No.:BCN5930
CAS No.:20575-57-9
- SB273005
Catalog No.:BCC6501
CAS No.:205678-31-5
- Coniferaldehyde
Catalog No.:BCN4899
CAS No.:20649-42-7
- Sinapaldehyde
Catalog No.:BCN4900
CAS No.:20649-43-8
- Carmichaenine A
Catalog No.:BCN7729
CAS No.:2065228-59-1
- Carmichaenine B
Catalog No.:BCN7733
CAS No.:2065228-60-4
- Carmichaenine C
Catalog No.:BCN7731
CAS No.:2065228-61-5
- Carmichaenine D
Catalog No.:BCN7732
CAS No.:2065228-62-6
- Carmichaenine E
Catalog No.:BCN7730
CAS No.:2065228-63-7
- 7-O-Methylporiol
Catalog No.:BCN3948
CAS No.:206560-99-8
- Pasiniazid
Catalog No.:BCC3835
CAS No.:2066-89-9
- Cannabichromene
Catalog No.:BCN4901
CAS No.:20675-51-8
- Ecliptasaponin D
Catalog No.:BCN2760
CAS No.:206756-04-9
- Pedatisectine F
Catalog No.:BCN4902
CAS No.:206757-32-6
Interaction of Rifampin and Darunavir-Ritonavir or Darunavir-Cobicistat In Vitro.[Pubmed:28193650]
Antimicrob Agents Chemother. 2017 Apr 24;61(5). pii: AAC.01776-16.
Treatment of HIV-infected patients coinfected with Mycobacterium tuberculosis is challenging due to drug-drug interactions (DDIs) between antiretrovirals (ARVs) and antituberculosis (anti-TB) drugs. The aim of this study was to quantify the effect of cobicistat (COBI) or ritonavir (RTV) in modulating DDIs between Darunavir (DRV) and rifampin (RIF) in a human hepatocyte-based in vitro model. Human primary hepatocyte cultures were incubated with RIF alone or in combination with either COBI or RTV for 3 days, followed by coincubation with DRV for 1 h. The resultant DRV concentrations were quantified by high-performance liquid chromatography with UV detection, and the apparent intrinsic clearance (CLint.app.) of DRV was calculated. Both RTV and COBI lowered the RIF-induced increases in CLint.app. in a concentration-dependent manner. Linear regression analysis showed that log10 RTV and log10 COBI concentrations were associated with the percent inhibition of RIF-induced elevations in DRV CLint.app., where beta was equal to -234 (95% confidence interval [CI] = -275 to -193; P < 0.0001) and -73 (95% CI = -89 to -57; P < 0.0001), respectively. RTV was more effective in lowering 10 muM RIF-induced elevations in DRV CLint.app. (half-maximal [50%] inhibitory concentration [IC50] = 0.025 muM) than COBI (IC50 = 0.223 muM). Incubation of either RTV or COBI in combination with RIF was sufficient to overcome RIF-induced elevations in DRV CLint.app., with RTV being more potent than COBI. These data provide the first in vitro experimental insight into DDIs between RIF and COBI-boosted or RTV-boosted DRV and will be useful to inform physiologically based pharmacokinetic (PBPK) models to aid in optimizing dosing regimens for the treatment of patients coinfected with HIV and M. tuberculosis.
Refining criteria for selecting candidates for a safe lopinavir/ritonavir or darunavir/ritonavir monotherapy in HIV-infected virologically suppressed patients.[Pubmed:28192453]
PLoS One. 2017 Feb 13;12(2):e0171611.
OBJECTIVE: The primary objective of this study was to estimate the incidence of treatment failure (TF) to protease inhibitor monotherapies (PI/r-MT) with lopinavir/ritonavir (LPV/r) or Darunavir/ritonavir (DRV/r). DESIGN: A multicenter cohort of HIV-infected patients with viral load (VL) 100 cells/muL) and residual viremia (aHR = 1.48 [95% CI: 1.01-2.17] vs. undetectable VL) were independently associated to TF. CONCLUSIONS: Residual viremia and nadir CD4+ counts <100 cells/muL should be regarded as the main factors to be taken into account before considering switching to a PI/r-MT.
A rapid validated UV-HPLC method for the simultaneous determination of the antiretroviral compounds darunavir and raltegravir in their dosage form.[Pubmed:28361525]
Rev Esp Quimioter. 2017 Jun;30(3):195-200. Epub 2017 Mar 29.
OBJECTIVE: A rapid, simple and sensitive high-performance liquid chromatography (HPLC) method with ultraviolet detection has been developed for quantification of Darunavir and raltegravir in their pharmaceutical dosage form. METHODS: The assay enables the measurement of both drugs with a linear calibration curve (R2= 0.999) over the concentration range 5-100 mg/L. The determination was performed on an analytical Tracer Excel 120 ODSB (15x0.4.6 cm) column at 35 masculineC. The selected wavelength was 254 nm. The mobile phase was a mixture of 0.037 M sodium dihydrogen phosphate buffer, acetonitrile and methanol (40:50:10, v/v/v) at a flow rate of 2.0 mL/min Nevirapine (50 mg/L) was used as internal standard. RESULTS: Accuracy, intra-day repeatability (n = 5), and inter-day precision (n = 3) were found to be satisfactory, being the accuracy from -4.33 to 3.88% and precisions were intra-day and inter-day, 0.25% and 4.42% respectively in case of Darunavir. Raltegravir intra-day and inter-day precisions lower of 1.01 and 2.36%, respectively and accuracy values bet from -4.02 to 1.06%. CONCLUSIONS: Determination of the Darunavir and raltegravir in their dosage form was done with a maximum deviation of 4%. This analytical method is rapid, easily implantable and offers good results.
TURQUOISE-I Part 1b: Ombitasvir/Paritaprevir/Ritonavir and Dasabuvir with Ribavirin for Hepatitis C Virus Infection in HIV-1 Coinfected Patients on Darunavir.[Pubmed:28329334]
J Infect Dis. 2017 Feb 15;215(4):599-605.
Background: Ombitasvir/paritaprevir/ritonavir with dasabuvir (OBV/PTV/r + DSV) +/- ribavirin (RBV) is approved for hepatitis C virus (HCV) genotype 1 (GT1) treatment in HIV-1 coinfected patients. In healthy controls, coadministration of OBV/PTV/r + DSV + Darunavir (DRV) lowered DRV trough concentration (Ctrough) levels. To assess the clinical significance of this change, TURQUOISE-I, Part 1b, evaluated the efficacy and safety of OBV/PTV/r + DSV + RBV in coinfected patients on stable, DRV-containing antiretroviral therapy (ART). Methods: Patients were HCV treatment-naive or interferon-experienced, had CD4+ lymphocyte count >/=200 cells/microL or >/=14%, and plasma HIV-1 RNA suppression on once-daily (QD) DRV-containing ART at screening. Patients were randomized to maintain DRV 800 mg QD or switch to twice-daily (BID) DRV 600 mg; all received OBV/PTV/r + DSV + RBV for 12 weeks. Results: Twenty-two patients were enrolled and achieved SVR12. No adverse events led to discontinuation. Coadministration had minimal impact on DRV maximum observed plasma concentration and area under the curve; DRV Ctrough levels were slightly lower with DRV QD and BID. No patient experienced plasma HIV-1 RNA >200 copies/mL during treatment. Conclusions: HCV GT1/HIV-1 coinfected patients on stable DRV-containing ART achieved 100% SVR12 while maintaining plasma HIV-1 RNA suppression. Despite DRV exposure changes, episodes of intermittent HIV-1 viremia were infrequent.


