ConiferaldehydeCAS# 20649-42-7 |
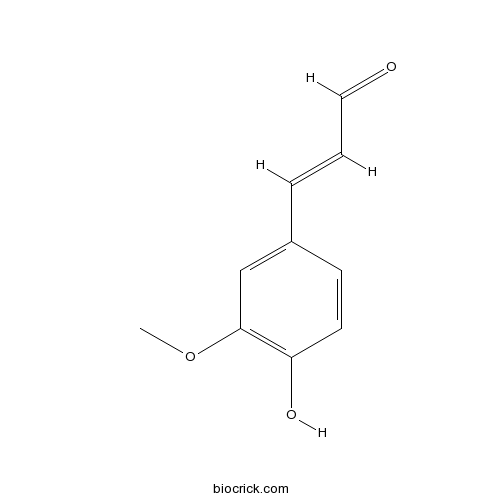
- Coniferylaldehydel
Catalog No.:BCX0648
CAS No.:458-36-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20649-42-7 | SDF | Download SDF |
| PubChem ID | 5280536 | Appearance | Powder |
| Formula | C10H10O3 | M.Wt | 178.2 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Synonyms | 3-(4-Hydroxy-3-Methoxyphenyl)-2-Propenal;trans-Coniferaldehyde;Coniferyl Aldehyde; 4-HYDROXY-3-METHOXYCINNAMALDEHYDE; 458-36-6;Ferulyl Aldehyde; Ferulaldehyde | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enal | ||
| SMILES | COC1=C(C=CC(=C1)C=CC=O)O | ||
| Standard InChIKey | DKZBBWMURDFHNE-NSCUHMNNSA-N | ||
| Standard InChI | InChI=1S/C10H10O3/c1-13-10-7-8(3-2-6-11)4-5-9(10)12/h2-7,12H,1H3/b3-2+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Coniferaldehyde exerts anti-inflammatory properties by inducing heme oxygenase-1(HO-1), it inhibits LPS-induced apoptosis through the PKC α/β II/Nrf-2/HO-1 dependent pathway in RAW264.7 macrophage cells. 2. Coniferaldehyde can significantly inhibit the growth of viability of strains of Oenococcus oeni . |
| Targets | NOS | COX | HO-1 | PKC | Nrf2 |

Coniferaldehyde Dilution Calculator

Coniferaldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6117 mL | 28.0584 mL | 56.1167 mL | 112.2334 mL | 140.2918 mL |
| 5 mM | 1.1223 mL | 5.6117 mL | 11.2233 mL | 22.4467 mL | 28.0584 mL |
| 10 mM | 0.5612 mL | 2.8058 mL | 5.6117 mL | 11.2233 mL | 14.0292 mL |
| 50 mM | 0.1122 mL | 0.5612 mL | 1.1223 mL | 2.2447 mL | 2.8058 mL |
| 100 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1223 mL | 1.4029 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Darunavir
Catalog No.:BCC3623
CAS No.:206361-99-1
- L-R4W2
Catalog No.:BCC5779
CAS No.:206350-79-0
- Monomelittoside
Catalog No.:BCN8509
CAS No.:20633-72-1
- Calycosin-7-O-beta-D-glucoside
Catalog No.:BCN5931
CAS No.:20633-67-4
- Encecalin
Catalog No.:BCN4898
CAS No.:20628-09-5
- CB30865
Catalog No.:BCC1457
CAS No.:206275-15-2
- Tenofovir hydrate
Catalog No.:BCC4261
CAS No.:206184-49-8
- Ergosterol peroxide
Catalog No.:BCN4897
CAS No.:2061-64-5
- Tetrahydromagnolol
Catalog No.:BCN8255
CAS No.:20601-85-8
- H-D-Asn-OH.H2O
Catalog No.:BCC2879
CAS No.:2058-58-4
- Oxytetracycline hydrochloride
Catalog No.:BCC9110
CAS No.:2058-46-0
- Calycosin
Catalog No.:BCN5930
CAS No.:20575-57-9
- Sinapaldehyde
Catalog No.:BCN4900
CAS No.:20649-43-8
- Carmichaenine A
Catalog No.:BCN7729
CAS No.:2065228-59-1
- Carmichaenine B
Catalog No.:BCN7733
CAS No.:2065228-60-4
- Carmichaenine C
Catalog No.:BCN7731
CAS No.:2065228-61-5
- Carmichaenine D
Catalog No.:BCN7732
CAS No.:2065228-62-6
- Carmichaenine E
Catalog No.:BCN7730
CAS No.:2065228-63-7
- 7-O-Methylporiol
Catalog No.:BCN3948
CAS No.:206560-99-8
- Pasiniazid
Catalog No.:BCC3835
CAS No.:2066-89-9
- Cannabichromene
Catalog No.:BCN4901
CAS No.:20675-51-8
- Ecliptasaponin D
Catalog No.:BCN2760
CAS No.:206756-04-9
- Pedatisectine F
Catalog No.:BCN4902
CAS No.:206757-32-6
- 3,7-Di-O-methylquercetin
Catalog No.:BCN6486
CAS No.:2068-02-2
Heat shock factor 1 inducers from the bark of Eucommia ulmoides as cytoprotective agents.[Pubmed:23847077]
Chem Biodivers. 2013 Jul;10(7):1322-7.
The barks of Eucommia ulmoides (Eucommiae Cortex, Eucommiaceae) have been used as a traditional medicine in Korea, Japan, and China to treat hypertension, reinforce the muscles and bones, and recover the damaged liver and kidney functions. Among these traditional uses, to establish the recovery effects on the damaged organs on the basis of phytochemistry, the barks of E. ulmoides have been investigated to afford three known phenolic compounds, Coniferaldehyde glucoside (1), bartsioside (2), and feretoside (3), which were found in the family Eucommiaceae for the first time. The compounds 1-3 were evaluated for their inducible activities on the heat shock factor 1 (HSF1), and heat shock proteins (HSPs) 27 and 70, along with four compounds, geniposide (4), geniposidic acid (5), pinoresinol diglucoside (6), and liriodendrin (7), which were previously reported from E. ulmoides. Compounds 1-7 increased expression of HSF1 by a factor of 1.214, 1.144, 1.153, 1.114, 1.159, 1.041, and 1.167 at 3 muM, respectively. Coniferaldehyde glucoside (1) showed the most effective increase of HSF1 and induced successive expressions of HSP27 and HSP70 in a dose-dependent manner without cellular cytotoxicity, suggesting a possible application as a HSP inducer to act as cytoprotective agent.
Ferulaldehyde Improves the Effect of Methotrexate in Experimental Arthritis.[Pubmed:29113115]
Molecules. 2017 Nov 6;22(11). pii: molecules22111911.
Methotrexate (MTX) is still the gold standard for treatment of rheumatoid arthritis (RA). The therapeutic efficacy of low-dose of MTX can be increased by its combination with a natural substance, ferulaldehyde (FRA). The aim of this study was to evaluate the effect FRA and MTX administered alone or in combination in adjuvant arthritis. The disease was induced to Lewis male rats by intradermal injection, which contains a suspension of heat-inactivated Mycobacterium butyricum in incomplete Freund's adjuvant. The experiment of 28 days included: healthy animals, arthritic animals, arthritic animals with administration of FRA at the oral daily dose of 15 mg/kg, arthritic animals with administration of MTX at the oral dose of 0.3 mg/kg twice a week, and arthritic animals administered with FRA and MTX. FRA in monotherapy decreased significantly only the level of interleukin-1beta (IL-1beta) and matrix metalloproteinase-9 in plasma. Combination of FRA and low-dose MTX was more effective than MTX alone when comparing body weight, hind paw volume, arthritic score, plasmatic levels of IL-1beta, activity of gamma-glutamyl transferase, and relative mRNA expression of IL-1beta in the spleen. Therefore, the combination treatment was the most effective. The obtained results are interesting for future possible innovative therapy of patients with RA.
Ferulaldehyde and lupeol as direct and indirect antimicrobial compounds from Cordia gilletii (Boraginaceae) root barks.[Pubmed:25026702]
Nat Prod Commun. 2014 May;9(5):619-22.
Cordia gilletii De Wild (Boraginaceae), a medicinal plant used against infectious diseases in the Democratic Republic of Congo, was investigated for direct and indirect antimicrobial properties. On one hand, the methanol extract is active against many pathogenic bacteria, including resistant strains. Its bio-guided fractionation led to the isolation of ferulaldehyde; this compound showed antimicrobial and antioxidant properties that may support the activity we observed for the methanol extract and some of the traditional uses of C. gilletii. On the other hand, the n-hexane extract of root barks possesses indirect antimicrobial properties, enhancing the activity of antibiotics against methicillin-resistant Staphylococcus aureus (MRSA). The fractionation of this extract led to the isolation of lupeol, which decreases the minimum inhibitory concentration of several antibiotics (4 to 8 fold) against MRSA and contributes to the effects observed for the raw n-hexane extract.
Identification of Escherichia coli biomarkers responsive to various lignin-hydrolysate compounds.[Pubmed:22445268]
Bioresour Technol. 2012 Jun;114:450-6.
Aberrations in the growth and transcriptome of Escherichia coli str. BL21(DE3) were determined when exposed to varying concentrations of ferulic acid (0.25-1 g/L), an aromatic carboxylic acid identified within lignin-cellulose hydrolysate samples. The expression of several individual genes (aaeA, aaeB, inaA and marA) was significantly induced, i.e., more than 4-fold, and thus these genes and the heat shock response gene htpG were selected as biomarkers to monitor E. coli's responses to five additional hydrolysate-related compounds, including vanillic acid, coumaric acid, 4-hydroxybenzoic acid, ferulaldehyde and furfural. While all of the biomarkers showed dose-dependent responses to most of the compounds, expression of aaeA and aaeB showed the greatest induction (5-30-fold) for all compounds tested except furfural. Lastly, the marA, inaA and htpG genes all showed higher expression levels when the culture was exposed to spruce hydrolysate samples, demonstrating the potential use of these genes as biomarkers.
Protective effects of ferulic acid and related polyphenols against glyoxal- or methylglyoxal-induced cytotoxicity and oxidative stress in isolated rat hepatocytes.[Pubmed:25446858]
Chem Biol Interact. 2015 Jun 5;234:96-104.
Glyoxal (GO) and methylglyoxal (MGO) cause protein and nucleic acid carbonylation and oxidative stress by forming reactive oxygen and carbonyl species which have been associated with toxic effects that may contribute to cardiovascular disease, complications associated with diabetes mellitus, Alzheimer's and Parkinson's disease. GO and MGO can be formed through oxidation of commonly used reducing sugars e.g., fructose under chronic hyperglycemic conditions. GO and MGO form advanced glycation end products which lead to an increased potential for developing inflammatory diseases. In the current study, we have investigated the protective effects of ferulic acid and related polyphenols e.g., caffeic acid, p-coumaric acid, methyl ferulate, ethyl ferulate, and ferulaldehyde on GO- or MGO-induced cytotoxicity and oxidative stress (ROS formation, protein carbonylation and mitochondrial membrane potential maintenance) in freshly isolated rat hepatocytes. To investigate and compare the protective effects of ferulic acid and related polyphenols against GO- or MGO-induced toxicity, five hepatocyte models were used: (a) control hepatocytes, (b) GSH-depleted hepatocytes, (c) catalase-inhibited hepatocytes, (d) aldehyde dehydrogenase (ALDH2)-inhibited hepatocytes, and (e) hepatocyte inflammation system (a non-toxic H2O2-generating system). All of the polyphenols tested significantly decreased GO- or MGO-induced cytotoxicity, ROS formation and improved mitochondrial membrane potential in these models. The rank order of their effectiveness was caffeic acid approximately ferulaldehyde>ferulic acid>ethyl ferulate>methyl ferulate>p-coumaric acid. Ferulic acid was found to decrease protein carbonylation in GSH-depleted hepatocytes. This study suggests that ferulic acid and related polyphenols can be used therapeutically to inhibit or decrease GO- or MGO-induced hepatotoxicity.
Suppressing LPS-induced early signal transduction in macrophages by a polyphenol degradation product: a critical role of MKP-1.[Pubmed:20884647]
J Leukoc Biol. 2011 Jan;89(1):105-11.
Macrophages represent the first defense line against bacterial infection and therefore, play a crucial role in early inflammatory response. In this study, we investigated the role of MAPKs and MKP-1 activation in regulation of an early inflammatory response in RAW 264.7 macrophage cells. We induced the inflammatory response by treating the macrophages with LPS and inhibited an early inflammatory response by using ferulaldehyde, a water-soluble end-product of dietary polyphenol degradation that we found previously to exert its beneficial anti-inflammatory effects during the early phase of in vivo inflammation. We found that LPS-induced ROS and nitrogen species formations were reduced by ferulaldehyde in a concentration-dependent manner, and ferulaldehyde protected mitochondria against LPS-induced rapid and massive membrane depolarization. LPS induced early suppression of MKP-1, which was accompanied by activation of JNK, ERK, and p38 MAPK. By reversing LPS-induced early suppression of MKP-1, ferulaldehyde diminished MAPK activation, thereby inhibiting NF-kappaB activation, mitochondrial depolarization, and ROS production. Taken together, our data suggest that ferulaldehyde exerts its early anti-inflammatory effect by preserving the mitochondrial membrane integrity and shifting the expression of MKP-1 forward in time in macrophages.
Effect of phenolic aldehydes and flavonoids on growth and inactivation of Oenococcus oeni and Lactobacillus hilgardii.[Pubmed:17993383]
Food Microbiol. 2008 Feb;25(1):105-12.
The aim of this work was to investigate the effect of wine phenolic aldehydes, flavonoids and tannins on growth and viability of strains of Oenococcus oeni and Lactobacillus hilgardii. Cultures were grown in ethanol-containing MRS/TJ medium supplemented with different concentrations of phenolic aldehydes or flavonoids and monitored spectrophotometrically. The effect of tannins was evaluated by monitoring the progressive inactivation of cells in ethanol-containing phosphate buffer supplemented with grape seed extracts with different molecular weight tannins. Of the phenolic aldehydes tested, sinapaldehyde, Coniferaldehyde, p-hydroxybenzaldehyde, 3,4-dihydroxybenzaldehyde and 3,4,5-trihydroxybenzaldehyde significantly inhibited the growth of O. oeni VF, while vanillin and syringaldehyde had no effect at the concentrations tested. Lact. hilgardii 5 was only inhibited by sinapaldehyde and Coniferaldehyde. Among the flavonoids, quercetin and kaempferol exerted an inhibitory effect especially on O. oeni VF. Myricetin and the flavan-3-ols studied (catechin and epicatechin) did not affect considerably the growth of both strains. Condensed tannins (particularly tetramers and pentamers) were found to strongly affect cell viability, especially in the case of O. oeni VF. In general, this strain was found to be more sensitive than Lact. hilgardii 5 to the phenolic compounds studied. This work contributes to the knowledge of the effect of different phenolic compounds on the activity of wine lactic acid bacteria, which, especially in the case of aldehydes and of different molecular weight fractions of tannins, is very scarce.
A new aldehyde compound from the fruit of Pandanus tectorius Parkinson ex Du Roi.[Pubmed:25674660]
Nat Prod Res. 2015;29(15):1437-41.
From the fruit of Pandanus tectorius Parkinson ex Du Roi, one new (1) and six known aldehyde compounds (2-7) were isolated by various chromatography methods. Based on their spectroscopic data, these compounds were identified as (Z)-4-hydroxy-3-(4-hydroxy-3-methylbut-2-en-1-yl) benzaldehyde (1), p-hydroxybenzaldehyde (2), syringaldehyde (3), (E)-ferulaldehyde (4), (E)-sinapinaldehyde (5), vanillin (6) and 5-hydroxymethylfurfual (7). The alpha-glucosidase inhibitory activity of all compounds was measured. The isolated compounds (1-6) showed better alpha-glucosidase inhibitory activity (IC50 values ranging from 36.5 to 192.4 muM) than the standard drug acarbose (IC50 = 214.5 muM).
Coniferaldehyde inhibits LPS-induced apoptosis through the PKC alpha/beta II/Nrf-2/HO-1 dependent pathway in RAW264.7 macrophage cells.[Pubmed:27770660]
Environ Toxicol Pharmacol. 2016 Dec;48:85-93.
Coniferaldehyde (CA) exerts anti-inflammatory properties by inducing heme oxygenase-1 (HO-1). To define the regulation mechanism by which CA induces a cytoprotective function and HO-1 expression, the up-stream regulations involved in the activation of nuclear transcription factor-erythroid 2-related factor (Nrf)-2/HO-1 pathway were investigated. CA dramatically increased the Nrf-2 nuclear translocation and HO-1 expression. Lipopolysaccharide (LPS)-induced expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2, and cell death were down-regulated by CA, which were reversed by inhibition of HO-1 activity. Furthermore, CA specifically enhanced the phosphorylation of protein kinase C (PKC) alpha/beta II. Selective inhibition of PKC alpha/beta II using Go6976 or siRNA abolished the CA-induced Nrf-2/HO-1 signaling, and consequently suppressed the cytoprotective activity of CA on the LPS-induced cell death. Together, our results elucidate the regulatory mechanism of PKC alpha/beta II as the upstream molecule of Nrf-2 required for HO-1 expression during CA-induced anti-inflammatory cytoprotective function in LPS stimulated macrophages.
Potential roles of chemical degradation in the biological activities of curcumin.[Pubmed:28138677]
Food Funct. 2017 Mar 22;8(3):907-914.
Substantial pre-clinical and human studies have shown that curcumin, a dietary compound from turmeric, has a variety of health-promoting biological activities. A better understanding of the biochemical mechanisms for the health-promoting effects of curcumin could facilitate the development of effective strategies for disease prevention. Recent studies have shown that in aqueous buffer, curcumin rapidly degrades and leads to formation of various degradation products. In this review, we summarized and discussed the biological activities of chemical degradation products of curcumin, including alkaline hydrolysis products (such as ferulic acid, vanillin, ferulaldehyde, and feruloyl methane), and autoxidation products (such as bicyclopentadione). Though many of these degradation products are biologically active, they are substantially less-active compared to curcumin, supporting that chemical degradation has a limited contribution to the biological activities of curcumin.


