EncecalinCAS# 20628-09-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20628-09-5 | SDF | Download SDF |
| PubChem ID | 114703 | Appearance | Oil |
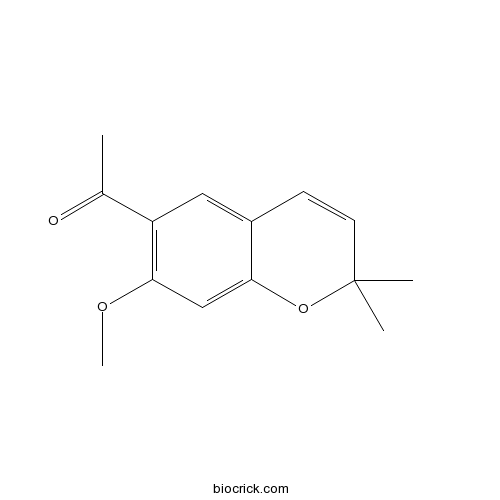
| Formula | C14H16O3 | M.Wt | 232.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(7-methoxy-2,2-dimethylchromen-6-yl)ethanone | ||
| SMILES | CC(=O)C1=C(C=C2C(=C1)C=CC(O2)(C)C)OC | ||
| Standard InChIKey | WXVLCNREBFDEKS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H16O3/c1-9(15)11-7-10-5-6-14(2,3)17-12(10)8-13(11)16-4/h5-8H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Encecalin and demethylencecalin are major phytotoxic compounds isolated from Helianthella quinquenervis (Hook) A Gray (Asteraceae), they inhibit photosystem II (from water to 2,5-dibromo-3-methyl-6-isopropyl-1,4 p-benzo-quinone). 2. Encecalin and eupatoriochromene retard seed germination and reduce radicle and hypocotyl growth of weed and crop plant seedlings. |
| Targets | ATPase |

Encecalin Dilution Calculator

Encecalin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3048 mL | 21.5239 mL | 43.0478 mL | 86.0956 mL | 107.6195 mL |
| 5 mM | 0.861 mL | 4.3048 mL | 8.6096 mL | 17.2191 mL | 21.5239 mL |
| 10 mM | 0.4305 mL | 2.1524 mL | 4.3048 mL | 8.6096 mL | 10.7619 mL |
| 50 mM | 0.0861 mL | 0.4305 mL | 0.861 mL | 1.7219 mL | 2.1524 mL |
| 100 mM | 0.043 mL | 0.2152 mL | 0.4305 mL | 0.861 mL | 1.0762 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CB30865
Catalog No.:BCC1457
CAS No.:206275-15-2
- Tenofovir hydrate
Catalog No.:BCC4261
CAS No.:206184-49-8
- Ergosterol peroxide
Catalog No.:BCN4897
CAS No.:2061-64-5
- Tetrahydromagnolol
Catalog No.:BCN8255
CAS No.:20601-85-8
- H-D-Asn-OH.H2O
Catalog No.:BCC2879
CAS No.:2058-58-4
- Oxytetracycline hydrochloride
Catalog No.:BCC9110
CAS No.:2058-46-0
- Calycosin
Catalog No.:BCN5930
CAS No.:20575-57-9
- SB273005
Catalog No.:BCC6501
CAS No.:205678-31-5
- Orexin B (human)
Catalog No.:BCC5765
CAS No.:205640-91-1
- Orexin A (human, rat, mouse)
Catalog No.:BCC5764
CAS No.:205640-90-0
- alpha-Chaconine
Catalog No.:BCN2162
CAS No.:20562-03-2
- alpha-Solanine
Catalog No.:BCN2701
CAS No.:20562-02-1
- Calycosin-7-O-beta-D-glucoside
Catalog No.:BCN5931
CAS No.:20633-67-4
- Monomelittoside
Catalog No.:BCN8509
CAS No.:20633-72-1
- L-R4W2
Catalog No.:BCC5779
CAS No.:206350-79-0
- Darunavir
Catalog No.:BCC3623
CAS No.:206361-99-1
- Coniferaldehyde
Catalog No.:BCN4899
CAS No.:20649-42-7
- Sinapaldehyde
Catalog No.:BCN4900
CAS No.:20649-43-8
- Carmichaenine A
Catalog No.:BCN7729
CAS No.:2065228-59-1
- Carmichaenine B
Catalog No.:BCN7733
CAS No.:2065228-60-4
- Carmichaenine C
Catalog No.:BCN7731
CAS No.:2065228-61-5
- Carmichaenine D
Catalog No.:BCN7732
CAS No.:2065228-62-6
- Carmichaenine E
Catalog No.:BCN7730
CAS No.:2065228-63-7
- 7-O-Methylporiol
Catalog No.:BCN3948
CAS No.:206560-99-8
Eupatoriochromene and encecalin, plant growth regulators from yellow starthistle (Centaurea solstitialis L.).[Pubmed:24272297]
J Chem Ecol. 1989 Jul;15(7):2073-87.
Two chromenes, eupatoriochromene (1) and Encecalin (2), have been isolated from yellow starthistle (Centaurea solstitialis L.). Both chromenes retard seed germination and reduce radicle and hypocotyl growth of weed and crop plant seedlings. In addition,1 increases adventitious root formation of mung bean cuttings.
Fate of the chromene encecalin in the interaction ofEncelia farinosa and its specialized herbivoreTrirhabda geminata.[Pubmed:24227488]
J Chem Ecol. 1996 Mar;22(3):491-8.
Leaf beetles of the speciesTrirhabda geminata are specialized herbivores that are able to feed on the chemically well-protected foliage of the desert sunflowerEncelia farinosa, which contains the insecticidal chromene derivative Encecalin. Chemical analysis of the beetles and their fecal excretions indicated that Encecalin is present only in the alimentary canal and is not absorbed across the gut membrane, as previously shown for other herbivorous insects (e.g., the Egyptian armyworm,Spodoptera littoralis) that are susceptible to this chromene derivative. Further differences betweenT. geminata and nonadapted insects were observed with regard to the metabolism of Encecalin. Whereas the Encecalin-resistent leaf beetles metabolize Encecalin mainly to encecalol by reduction of the acetyl group, susceptible insects, such as larvae ofS. littoralis, metabolize Encecalin mainly by exoxidation of the 3,4 double bond, which creates a powerful alkylating agent and is responsible for the toxicity of Encecalin. Reductive rather than oxidative metabolism of Encecalin therefore seems important for the resistance ofT. geminata against the chemical defense of their host plantE. farinosa.


