CB30865Nampt inhibitor CAS# 206275-15-2 |
- CI994 (Tacedinaline)
Catalog No.:BCC2159
CAS No.:112522-64-2
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 206275-15-2 | SDF | Download SDF |
| PubChem ID | 9871194 | Appearance | Powder |
| Formula | C26H22BrN5O2 | M.Wt | 516.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ZM 242421 | ||
| Solubility | DMSO : 33.33 mg/mL (64.54 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
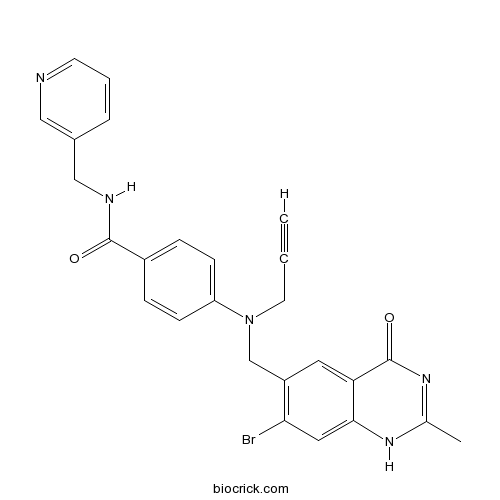
| Chemical Name | 4-[(7-bromo-2-methyl-4-oxo-1H-quinazolin-6-yl)methyl-prop-2-ynylamino]-N-(pyridin-3-ylmethyl)benzamide | ||
| SMILES | CC1=NC(=O)C2=CC(=C(C=C2N1)Br)CN(CC#C)C3=CC=C(C=C3)C(=O)NCC4=CN=CC=C4 | ||
| Standard InChIKey | SHNBLWMBWXIKMR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H22BrN5O2/c1-3-11-32(16-20-12-22-24(13-23(20)27)30-17(2)31-26(22)34)21-8-6-19(7-9-21)25(33)29-15-18-5-4-10-28-14-18/h1,4-10,12-14H,11,15-16H2,2H3,(H,29,33)(H,30,31,34) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CB30865(ZM 242421) is a potent inhibitor of Nampt , an enzyme present in the NAD biosynthetic pathway.
IC50 value:
Target: Nampt
Cancer cells develop dependence on Nampt due to increased energy requirements and the elevated activity of NAD consuming enzymes such as sirtuins and mono and poly(ADP-ribose) polymerases (PARPs). These findings suggest new chemical starting points for Nampt inhibitors and further implicate this enzyme as a target in cancer. References: | |||||

CB30865 Dilution Calculator

CB30865 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9365 mL | 9.6826 mL | 19.3652 mL | 38.7304 mL | 48.413 mL |
| 5 mM | 0.3873 mL | 1.9365 mL | 3.873 mL | 7.7461 mL | 9.6826 mL |
| 10 mM | 0.1937 mL | 0.9683 mL | 1.9365 mL | 3.873 mL | 4.8413 mL |
| 50 mM | 0.0387 mL | 0.1937 mL | 0.3873 mL | 0.7746 mL | 0.9683 mL |
| 100 mM | 0.0194 mL | 0.0968 mL | 0.1937 mL | 0.3873 mL | 0.4841 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 2.8 nM for W1L2; 156 nM for isolated mammalian TS
CB30865 is a highly potent cytotoxic agent. The compound inhibits isolated mammalian thymidylate synthase (TS), but this inhibition is insufficient to account for its cellular toxicity. Thymidylate synthase (TS) is a critical enzyme in the de novo synthesis of thymidylate (dTTP) and has long been recognized as a target for chemotherapeutic intervention.
In vitro: CB30865 was the most potent growth inhibitory agent (IC50 values in the range 1–100 nM for several mouse and human cell types). Against W1L2 cells, the analogues of CB300179 and CB300189 demonstrated reduced potency in the presence of exogenous thymidine (dThd), and against a W1L2:C1 TS overproducing cell line. In contrast, CB30865 retained activity in these systems. No cell cycle redistribution was observed following exposure (4–48 h) to an equitoxic concentration of CB30865 [1].
In vivo: The in vivo evaluation of CB30865 was hampered because of its low aqueous solubility (<1 μM at pH 6). This low aqueous solubility of CB30865 initiated a search for more water-soluble analogues to allow the in vivo evaluation of this class of compounds. It was envisaged that aqueous solubility could be increased by the introduction of amino functionalities (e.g., N-methylpiperazine) at the 2-position of the quinazolin-4-one ring [2].
Clinical trial: No clinical data are available
References:
[1] Bavetsias V, Skelton LA, Yafai F, Mitchell F, Wilson SC, Allan B, Jackman AL. The design and synthesis of water-soluble analogues of CB30865, a quinazolin-4-one-based antitumor agent. J Med Chem. 2002;45(17):3692-702.
[2] Skelton LA, Ormerod MG, Titley J, Kimbell R, Brunton LA, Jackman AL. A novel class of lipophilic quinazoline-based folic acid analogues: cytotoxic agents with a folate-independent locus. Br J Cancer. 1999;79(11-12):1692-701.
- Tenofovir hydrate
Catalog No.:BCC4261
CAS No.:206184-49-8
- Ergosterol peroxide
Catalog No.:BCN4897
CAS No.:2061-64-5
- Tetrahydromagnolol
Catalog No.:BCN8255
CAS No.:20601-85-8
- H-D-Asn-OH.H2O
Catalog No.:BCC2879
CAS No.:2058-58-4
- Oxytetracycline hydrochloride
Catalog No.:BCC9110
CAS No.:2058-46-0
- Calycosin
Catalog No.:BCN5930
CAS No.:20575-57-9
- SB273005
Catalog No.:BCC6501
CAS No.:205678-31-5
- Orexin B (human)
Catalog No.:BCC5765
CAS No.:205640-91-1
- Orexin A (human, rat, mouse)
Catalog No.:BCC5764
CAS No.:205640-90-0
- alpha-Chaconine
Catalog No.:BCN2162
CAS No.:20562-03-2
- alpha-Solanine
Catalog No.:BCN2701
CAS No.:20562-02-1
- Oxibendazole
Catalog No.:BCC4818
CAS No.:20559-55-1
- Encecalin
Catalog No.:BCN4898
CAS No.:20628-09-5
- Calycosin-7-O-beta-D-glucoside
Catalog No.:BCN5931
CAS No.:20633-67-4
- Monomelittoside
Catalog No.:BCN8509
CAS No.:20633-72-1
- L-R4W2
Catalog No.:BCC5779
CAS No.:206350-79-0
- Darunavir
Catalog No.:BCC3623
CAS No.:206361-99-1
- Coniferaldehyde
Catalog No.:BCN4899
CAS No.:20649-42-7
- Sinapaldehyde
Catalog No.:BCN4900
CAS No.:20649-43-8
- Carmichaenine A
Catalog No.:BCN7729
CAS No.:2065228-59-1
- Carmichaenine B
Catalog No.:BCN7733
CAS No.:2065228-60-4
- Carmichaenine C
Catalog No.:BCN7731
CAS No.:2065228-61-5
- Carmichaenine D
Catalog No.:BCN7732
CAS No.:2065228-62-6
- Carmichaenine E
Catalog No.:BCN7730
CAS No.:2065228-63-7
Cell cycle effects of CB30865, a lipophilic quinazoline-based analogue of the antifolate thymidylate synthase inhibitor ICI 198583 with an undefined mechanism of action.[Pubmed:9725559]
Cytometry. 1998 Sep 1;33(1):56-66.
CB30865 (p-[N-(7-bromo-3,4-dihydro-2-methyl-4-oxoquinazolin-6-ylmethyl+ ++)-N-(prop-2-ynyl)amino]-N-(3-pyridylmethyl)benzamide) is a quinazoline-based pyridine-containing compound that emerged from a programme aimed at the development of thymidylate synthase (TS) inhibitors as anticancer agents. Its structure is based on the antifolate ICI 198583, but with a pyridine ring replacing the glutamate. Despite its structure, CB30865 does not act in vitro via inhibition of TS or, apparently, other known folate-dependent pathways, and extensive mechanistic studies suggest that it acts via a novel locus with respect to conventional antitumour agents. However, CB30865 is highly potent against a variety of human tumour cell lines (e.g., 50%-inhibitory concentration [IC50] values in the 1-10 nM range). Thus, the cell cycle effects of CB30865 were investigated. DNA histogram analysis of W1L2 human lymphoblastoid, L1210 murine leukaemia, and CH1 human ovarian cells (propidium iodide staining) has demonstrated that CB30865 does not cause a phase-specific arrest at concentrations that have been shown to inhibit colony formation. This is unexpected for an anticancer agent. In W1L2 cells, using bromodeoxyuridine (BrdUrd) labelling and bivariate Hoechst/ propidium iodide staining, it was revealed that 0.003-0.15 microM CB30865 (1-50 x 72 h IC50) caused cells to arrest in all phases of the cell cycle simultaneously after 20-24 h exposure. This effect was also observed in CH1 and L1210 cells, though the arrest was at slightly different times. Thus, using this technique, it has been demonstrated that CB30865 induces an unusual and delayed cell cycle arrest, which provides further evidence for a novel locus of action for this compound.
Chemical proteomics identifies Nampt as the target of CB30865, an orphan cytotoxic compound.[Pubmed:20609415]
Chem Biol. 2010 Jun 25;17(6):659-64.
Drug discovery based on cellular phenotypes is impeded by the challenge of identifying the molecular target. To alleviate this problem, we developed a chemical proteomic process to identify cellular proteins that bind to small molecules. CB30865 is a potent (subnanomolar) and selective cytotoxic compound of previously unknown mechanism of action. By combining chemical proteomics with biochemical and cellular pharmacology we have determined that CB30865 cytotoxicity is due to subnanomolar inhibition of nicotinamide phosphoribosyltransferase (Nampt), an enzyme present in the NAD biosynthetic pathway. Cancer cells develop dependence on Nampt due to increased energy requirements and the elevated activity of NAD consuming enzymes such as sirtuins and mono and poly(ADP-ribose) polymerases (PARPs). These findings suggest new chemical starting points for Nampt inhibitors and further implicate this enzyme as a target in cancer.
The design and synthesis of water-soluble analogues of CB30865, a quinazolin-4-one-based antitumor agent.[Pubmed:12166942]
J Med Chem. 2002 Aug 15;45(17):3692-702.
4-[N-[7-Bromo-2-methyl-4-oxo-3,4-dihydroquinazolin-6-ylmethyl]-N-(prop-2-ynyl)ami no]-N-(3-pyridylmethyl)benzamide (CB30865) is a quinazolin-4-one antitumor agent whose high growth-inhibitory activity (W1L2 IC(50) = 2.8 +/- 0.50 nM) is believed to have a folate-independent locus of action. In addition, CB30865 represents a class of compounds with unique biochemical characteristics such as a delayed, non-phase specific, cell-cycle arrest. The low aqueous solubility of CB30865 prompted a search for more water-soluble analogues for in vivo evaluation of this class of compounds. It was thought that aqueous solubility could be increased by the introduction of amino functionalities at the 2-position of the quinazolin-4-one ring. A variety of compounds (5a-j, 31a-c, 32, and 33) were synthesized in a linear fashion starting from 3-chloro-4-methylaniline. Most of these compounds (e.g., 5a, 5b, 5g) were significantly more water-soluble than CB30865 (636 microM for 5a at pH 6 and 992 microM for 5g at pH 6). In addition, some of them were up to 6-fold more cytotoxic than CB30865 (e.g., for 5a, W1L2 IC(50) = 0.49 +/- 0.24 nM) and retained its novel biochemical characteristics.


