CilengitideIntegrin inhibitor for αvβ3 and αvβ5 CAS# 188968-51-6 |
- Cyclo (-RGDfK)
Catalog No.:BCC3590
CAS No.:161552-03-0
- BIO 1211
Catalog No.:BCC3945
CAS No.:187735-94-0
- TR-14035
Catalog No.:BCC4266
CAS No.:232271-19-1
- BIO 5192
Catalog No.:BCC8002
CAS No.:327613-57-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 188968-51-6 | SDF | Download SDF |
| PubChem ID | 176873 | Appearance | Powder |
| Formula | C27H40N8O7 | M.Wt | 588.66 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | EMD121974 | ||
| Solubility | DMSO : ≥ 44 mg/mL (74.75 mM) H2O : ≥ 32 mg/mL (54.36 mM) *"≥" means soluble, but saturation unknown. | ||
| Sequence | RGDFV (Modifications: Phe-4 = D-Phe, Val-5 = Me-Val, Cyclizedl) | ||
| Chemical Name | 2-[(2S,5R,8S,11S)-5-benzyl-11-[3-(diaminomethylideneamino)propyl]-7-methyl-3,6,9,12,15-pentaoxo-8-propan-2-yl-1,4,7,10,13-pentazacyclopentadec-2-yl]acetic acid | ||
| SMILES | CC(C)C1C(=O)NC(C(=O)NCC(=O)NC(C(=O)NC(C(=O)N1C)CC2=CC=CC=C2)CC(=O)O)CCCN=C(N)N | ||
| Standard InChIKey | AMLYAMJWYAIXIA-VWNVYAMZSA-N | ||
| Standard InChI | InChI=1S/C27H40N8O7/c1-15(2)22-25(41)33-17(10-7-11-30-27(28)29)23(39)31-14-20(36)32-18(13-21(37)38)24(40)34-19(26(42)35(22)3)12-16-8-5-4-6-9-16/h4-6,8-9,15,17-19,22H,7,10-14H2,1-3H3,(H,31,39)(H,32,36)(H,33,41)(H,34,40)(H,37,38)(H4,28,29,30)/t17-,18-,19+,22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective inhibitor of integrins αvβ3 and αvβ5 (IC50 values are 4.1 and 70 nM, respectively). Exhibits ~10-fold selectivity over gpIIb/IIIa. Increases endothelial monolayer permeability. Also exhibits antiangiogenic activity. |

Cilengitide Dilution Calculator

Cilengitide Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
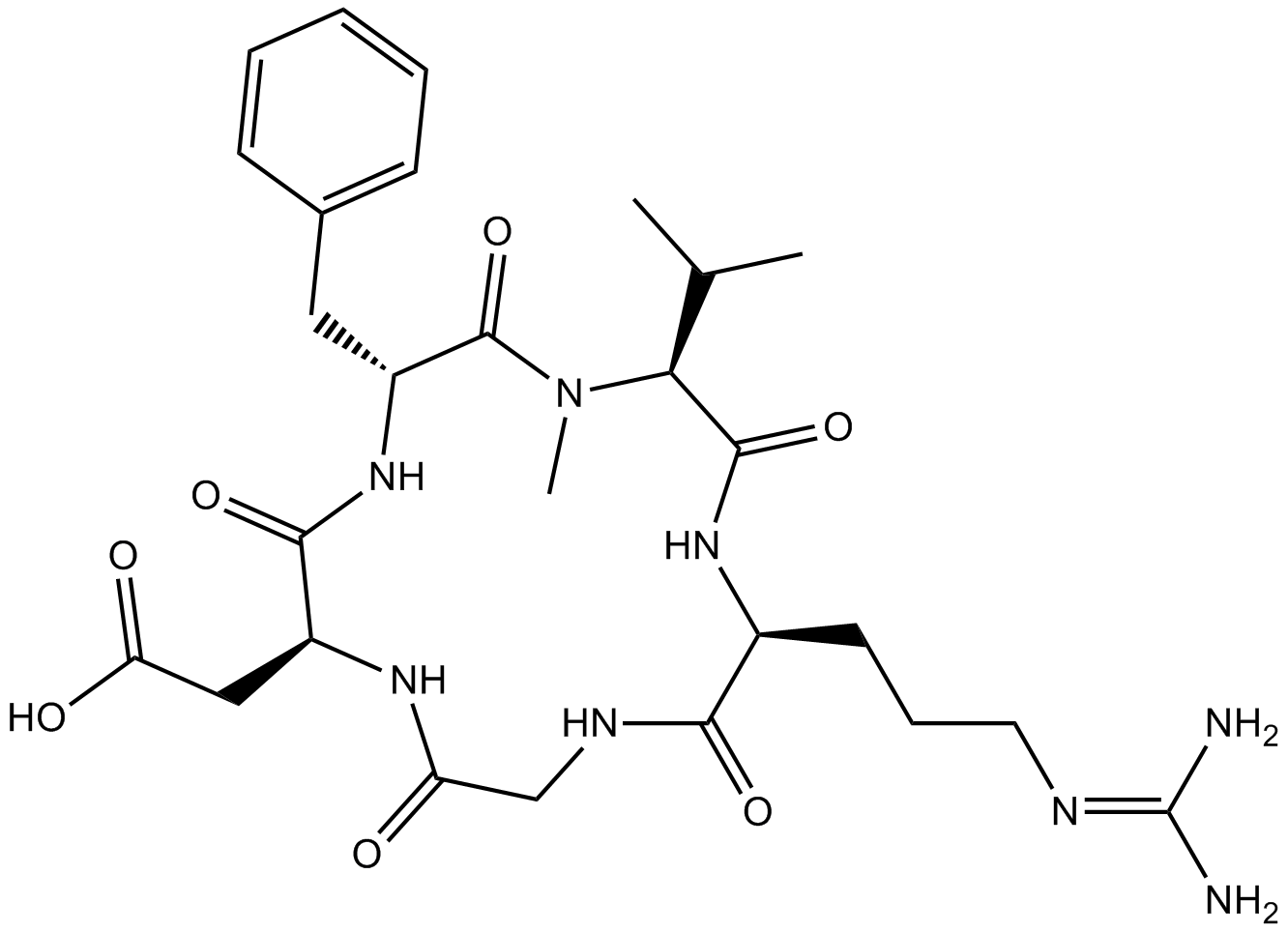
Cilengitide is a cyclic RGD pentapeptide [Arg-Gly-Asp-DPhe-(NMeVal)], and a potent αvβ3 and αvβ5 integrin inhibitor to block integrin-mediated adhesion and migration. It can directly bind αvβ3 integrin. Its IC50 is 250 nM as an αvβ3 inhibitor [1] [2] [3] [4].
Integrins are named for a family including 24 transmembrane heterodimer receptors that are composed of paired alpha and beta chains. These receptors can integrate extracellular and intracellular activities. Consequently, they can regulate tumor angiogenesis, migration and invasion [2].
When treated with cilengitide, a significant dose-dependent reduction of proliferation was noted (p<0.0002) in cell lines developed through 28 d of expansion and hence 14 d of differentiation culture of CD133+ stem cells (with VEGF, SCGF and FLT3L, to prepare CD133+ EPCs, i.e. endothelial progenitor cells). When treated with cilengitide after withdrawal of FLT3L and SCGF, a dose-dependent decrease of adherent cells was noted in EPCs after 7 and 14 days (p<0.03). Compared with which on EPC proliferation, the inhibitory effect of cilengitide on endothelial cell attachment was more pronounced [3].
Therapy with cilengitide intraperitoneally 5 times per week between days 1 and 30 after injection of MDA-MB-231 cells (105), volumes of osteolytic lesions(OL) and soft tissue components(SC) were significantly reduced on days 30 and 35 in rats, compared with untreated nude rats (p<0.05) [5].
References:
[1]. Carlos Mas-Moruno, Florian Rechenmacher and Horst Kessler. Cilengitide: The First Anti-Angiogenic Small Molecule Drug Candidate. Design, Synthesis and Clinical Evaluation. Anti-Cancer Agents in Medicinal Chemistry, 2010, 10(10): 753-768.
[2]. David A Reardon, L Burt Nabors, Roger Stupp, et al. Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drugs, 2008, 17(8):1225-1235.
[3]. Sonja Loges, Martin Butzal, Jasmin Otten, et al. Cilengitide inhibits proliferation and differentiation of human endothelial progenitor cells in vitro. Biochemical and Biophysical Research Communications, 2007, 357: 1016-1020.
[4]. Despoina Sykoutri, Nisha Geetha, Silvia Hayer, et al. αvβ3 Integrin Inhibition with Cilengitide both Prevents and Treats Collagen Induced Arthritis. Ann Rheum Dis, 2013, 72(Suppl 1):A1-A88.
[5]. Maren Bretschi, Maximilian Merz, Dorde Komljenovic, et al. Cilengitide inhibits metastatic bone colonization in a nude rat model. Oncology Reports, 2011, 26:843-851.
- L 760735
Catalog No.:BCC7840
CAS No.:188923-01-5
- Junipediol B 8-O-glucoside
Catalog No.:BCN4022
CAS No.:188894-19-1
- Methyl tanshinonate
Catalog No.:BCN2553
CAS No.:18887-19-9
- Hydroxytanshinone IIA
Catalog No.:BCN2497
CAS No.:18887-18-8
- DMA
Catalog No.:BCC1532
CAS No.:188860-26-6
- HX 630
Catalog No.:BCC6083
CAS No.:188844-52-2
- HX 531
Catalog No.:BCC6082
CAS No.:188844-34-0
- Streptozotocin
Catalog No.:BCN3834
CAS No.:18883-66-4
- 8-Glucosyl-5,7-dihydroxy-2-(1-methylpropyl)chromone
Catalog No.:BCN7505
CAS No.:188818-27-1
- SC 560
Catalog No.:BCC7111
CAS No.:188817-13-2
- N-Acetylcaprolactam
Catalog No.:BCC9081
CAS No.:1888-91-1
- 4,5-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6492
CAS No.:188742-80-5
- Melilotigenin C
Catalog No.:BCN1165
CAS No.:188970-21-0
- 1-(4-Hydroxy-2,2-dimethylchroman-6-yl)ethanone
Catalog No.:BCN7710
CAS No.:1890153-71-5
- Corynantheine
Catalog No.:BCN3746
CAS No.:18904-54-6
- NGB 2904
Catalog No.:BCC7435
CAS No.:189061-11-8
- [Ala92]-p16 (84-103)
Catalog No.:BCC5837
CAS No.:189064-08-2
- Oroselol
Catalog No.:BCN3907
CAS No.:1891-25-4
- Hulupinic acid
Catalog No.:BCN8019
CAS No.:1891-42-5
- Salmefamol
Catalog No.:BCC1919
CAS No.:18910-65-1
- Bruceantinoside C
Catalog No.:BCN1166
CAS No.:112899-35-1
- Fas C- Terminal Tripeptide
Catalog No.:BCC1019
CAS No.:189109-90-8
- 3'-O-Methylmurraol
Catalog No.:BCN7471
CAS No.:1891097-17-8
- Naringin dihydrochalcone
Catalog No.:BCN2579
CAS No.:18916-17-1
Cilengitide and Cetuximab Reduce Cytokine Production and Colony Formation of Head and Neck Squamous Cell Carcinoma Cells Ex Vivo.[Pubmed:28179297]
Anticancer Res. 2017 Feb;37(2):521-527.
BACKGROUND/AIM: To analyze ex vivo effects of combined targeting of the epidermal growth factor-receptor (EGFR) by cetuximab (E) plus alphaVbeta3 and alphaVbeta5 integrins by Cilengitide (Cil) on colony formation of epithelial cells (CFec) and release of pro-angiogenetic and pro-inflammatory cytokines in head and neck squamous cell carcinoma (HNSCC) cells. MATERIALS AND METHODS: Collagenase-IV digests of 43 histopathological confirmed HNSCC cases were seeded into laminin-coated 96-well plates containing E, Cil, or Cil+E in final concentrations of 66.7 mug/ml, 10 muM, and 10 muM+66.7 mug/ml, respectively. Following the FLAVINO-assay protocol, supernatants were harvested after 3 days and adherent cells fixed in ethanol. Counting of CFec was facilitated by FITC-labeled pan-cytokeratin antibodies. Out of 43 HNSCC cases, 39 had adherent growth (mean CFec>/=4/well in triplicate controls). Cytokines in supernatants were measured using ELISA were interleukin 6 (IL-6), monocyte chemoattractant protein 1 (MCP-1) and vascular endothelial growth factor A (VEGFA). RESULTS: CFec on laminin was significantly reduced by Cil, E, and Cil+E. Cytokine measurements also revealed significant suppression of MCP-1, IL-6 and VEGFA. The strongest suppression of CFec, MCP-1 and VEGFA release was exerted by Cil and E combined. Efficacy of Cil+E exceeded those of the solely applied pharmaceutics but failed regarding significant synergism of both treatments as E was unable to significantly boost the effects of Cil. In contrast, IL-6 release was significantly suppressed by E but not by Cil, while their combination strongly reduced it. CONCLUSION: Combined targeting of EGFR and integrins with E and Cil heightens their suppressive effects regarding CFec as well as release of pro-angiogenetic and pro-inflammatory cytokines.
Cilengitide with metronomic temozolomide, procarbazine, and standard radiotherapy in patients with glioblastoma and unmethylated MGMT gene promoter in ExCentric, an open-label phase II trial.[Pubmed:26935578]
J Neurooncol. 2016 May;128(1):163-171.
Newly diagnosed glioblastoma multiforme with unmethylated MGMT promoter has a poor prognosis, with a median survival of 12 months. This phase II study investigated the efficacy and safety of combining the selective integrin inhibitor Cilengitide with a combination of metronomic temozolomide and procarbazine for these patients. Eligible patients (newly diagnosed, histologically confirmed supratentorial glioblastoma with unmethylated MGMT promoter) were entered into this multicentre study. Cilengitide (2000 mg IV twice weekly) was commenced 1 week prior to radiotherapy combined with daily temozolomide (60 mg/m(2)) and procarbazine (50 or 100 mg) and, after 4 weeks' break, followed by six adjuvant cycles of temozolomide (50-60 mg/m(2)) and procarbazine (50 or 100 mg) on days 1-20, every 28 days. Cilengitide was continued for up to 12 months or until disease progression or unacceptable toxicity. The primary endpoint for efficacy was a 12-month overall survival rate of 65 %. Twenty-nine patients completed study treatment. Sixteen patients survived for 12 months or more, an overall survival rate of 55 %. The median overall survival was 14.5 months (95 % CI 11.1-19.6) and the median progression-free survival was 7.4 months (95 % CI 6.1-8). Cilengitide combined with metronomic temozolomide and procarbazine in MGMT-promoter unmethylated glioblastoma did not improve survival compared with historical data and does not warrant further investigation.
In vitro and in vivo drug disposition of cilengitide in animals and human.[Pubmed:27069630]
Pharmacol Res Perspect. 2016 Mar 17;4(2):e00217.
Cilengitide is very low permeable (1.0 nm/sec) stable cyclic pentapeptide containing an Arg-Gly-Asp motif responsible for selective binding to alphavbeta3 and alphavbeta5 integrins administered intravenously (i.v.). In vivo studies in the mouse and Cynomolgus monkeys showed the major component in plasma was unchanged drug (>85%). These results, together with the absence of metabolism in vitro and in animals, indicate minimal metabolism in both species. The excretion of [(14)C]-Cilengitide showed profound species differences, with a high renal excretion of the parent drug observed in Cynomolgus monkey (50% dose), but not in mouse (7 and 28%: m/f). Consistently fecal (biliary) secretion was high in mouse (87 and 66% dose: m/f) but low in Cynomolgus monkey (36.5%). Human volunteers administrated with [(14)C]-Cilengitide showed that most of the dose was recovered in urine as unchanged drug (77.5%, referred to Becker et al. 2015), indicating that the Cynomolgus monkey was the closer species to human. In order to better understand the species difference between human and mouse, the hepatobiliary disposition of [(14)C]-Cilengitide was determined in sandwich-cultured hepatocytes. Cilengitide exhibited modest biliary efflux (30-40%) in mouse, while in human hepatocytes this was negligible. Furthermore, it was confirmed that the uptake of Cilengitide into human hepatocytes was minor and appeared to be passive. In summary, the extent of renal and biliary secretion of Cilengitide appears to be highly species specific and is qualitatively well explained using sandwich hepatocyte culture models.
Cilengitide in newly diagnosed glioblastoma: biomarker expression and outcome.[Pubmed:26918452]
Oncotarget. 2016 Mar 22;7(12):15018-32.
Integrins alphavbeta3 and alphavbeta5 regulate angiogenesis and invasiveness in cancer, potentially by modulating activation of the transforming growth factor (TGF)-beta pathway. The randomized phase III CENTRIC and phase II CORE trials explored the integrin inhibitor Cilengitide in patients with newly diagnosed glioblastoma with versus without O6-methylguanine DNA methyltransferase (MGMT) promoter methylation. These trials failed to meet their primary endpoints.Immunohistochemistry was used to assess the levels of the target integrins of Cilengitide, alphavbeta3 and alphavbeta5 integrins, of alphavbeta8 and of their putative target, phosphorylation of SMAD2, in tumor tissues from CENTRIC (n=274) and CORE (n=224).alphavbeta3 and alphavbeta5 expression correlated well in tumor and endothelial cells, but showed little association with alphavbeta8 or pSMAD2 levels. In CENTRIC, there was no interaction between the biomarkers and treatment for prediction of outcome. In CORE, higher alphavbeta3 levels in tumor cells were associated with improved progression-free survival by central review and with improved overall survival in patients treated with Cilengitide.Integrins alphavbeta3, alphavbeta5 and alphavbeta8 are differentially expressed in glioblastoma. Integrin levels do not correlate with the activation level of the canonical TGF-beta pathway. alphavbeta3 integrin expression may predict benefit from integrin inhibition in patients with glioblastoma lacking MGMT promoter methylation.
The integrin antagonist cilengitide activates alphaVbeta3, disrupts VE-cadherin localization at cell junctions and enhances permeability in endothelial cells.[Pubmed:19212436]
PLoS One. 2009;4(2):e4449.
Cilengitide is a high-affinity cyclic pentapeptdic alphaV integrin antagonist previously reported to suppress angiogenesis by inducing anoikis of endothelial cells adhering through alphaVbeta3/alphaVbeta5 integrins. Angiogenic endothelial cells express multiple integrins, in particular those of the beta1 family, and little is known on the effect of Cilengitide on endothelial cells expressing alphaVbeta3 but adhering through beta1 integrins. Through morphological, biochemical, pharmacological and functional approaches we investigated the effect of Cilengitide on alphaVbeta3-expressing human umbilical vein endothelial cells (HUVEC) cultured on the beta1 ligands fibronectin and collagen I. We show that Cilengitide activated cell surface alphaVbeta3, stimulated phosphorylation of FAK (Y(397) and Y(576/577)), Src (S(418)) and VE-cadherin (Y(658) and Y(731)), redistributed alphaVbeta3 at the cell periphery, caused disappearance of VE-cadherin from cellular junctions, increased the permeability of HUVEC monolayers and detached HUVEC adhering on low-density beta1 integrin ligands. Pharmacological inhibition of Src kinase activity fully prevented Cilengitide-induced phosphorylation of Src, FAK and VE-cadherin, and redistribution of alphaVbeta3 and VE-cadherin and partially prevented increased permeability, but did not prevent HUVEC detachment from low-density matrices. Taken together, these observations reveal a previously unreported effect of Cilengitide on endothelial cells namely its ability to elicit signaling events disrupting VE-cadherin localization at cellular contacts and to increase endothelial monolayer permeability. These effects are potentially relevant to the clinical use of Cilengitide as anticancer agent.
CYR61 and alphaVbeta5 integrin cooperate to promote invasion and metastasis of tumors growing in preirradiated stroma.[Pubmed:18794119]
Cancer Res. 2008 Sep 15;68(18):7323-31.
Radiotherapy is widely used to treat human cancer. Patients locally recurring after radiotherapy, however, have increased risk of metastatic progression and poor prognosis. The clinical management of postradiation recurrences remains an unresolved issue. Tumors growing in preirradiated tissues have an increased fraction of hypoxic cells and are more metastatic, a condition known as tumor bed effect. The transcription factor hypoxia inducible factor (HIF)-1 promotes invasion and metastasis of hypoxic tumors, but its role in the tumor bed effect has not been reported. Here, we show that tumor cells derived from SCCVII and HCT116 tumors growing in a preirradiated bed, or selected in vitro through repeated cycles of severe hypoxia, retain invasive and metastatic capacities when returned to normoxia. HIF activity, although facilitating metastatic spreading of tumors growing in a preirradiated bed, is not essential. Through gene expression profiling and gain- and loss-of-function experiments, we identified the matricellular protein CYR61 and alphaVbeta5 integrin as proteins cooperating to mediate these effects. The anti-alphaV integrin monoclonal antibody 17E6 and the small molecular alphaVbeta3/alphaVbeta5 integrin inhibitor EMD121974 suppressed invasion and metastasis induced by CYR61 and attenuated metastasis of tumors growing within a preirradiated field. These results represent a conceptual advance to the understanding of the tumor bed effect and identify CYR61 and alphaVbeta5 integrin as proteins that cooperate to mediate metastasis. They also identify alphaV integrin inhibition as a potential therapeutic approach for preventing metastasis in patients at risk for postradiation recurrences.


